4761
Views & Citations3761
Likes & Shares
Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) are two types of lipid-nanoparticles that have drawn a lot of attention lately because they are effective, biodegradable, biocompatible, and non-toxic carriers with a range of useful qualities for dermal application of pharmaceuticals and cosmetics. Several surfactants stabilize the "solid lipid" core of SLN emulsion spheres, which have an average diameter of 10–1000 nm [4].
The liquid lipid component in NLC addresses the drawbacks of SLN by preventing the solid lipid matrix from crystallizing into a perfect matrix during storage and by enhancing the absorption of active substances [5]. Because NLC is made up of a combination of liquid and solid lipids,
its crystalline structure is defective, allowing more space between the lipid chains and the matrix [6].
Many disorders, some of which are potentially blinding, can affect the eye. Treating these diverse conditions has never been easy for medical professionals or the pharmaceutical business, especially when it comes to dosing and delivery methods. Recent advancements have been made in the difficult-to-treat area of the eye, drug administration [7]. The eye is a relatively isolated organ in the body, with several defenses and mechanisms that prohibit the consumption of foreign substances. Polysaccharides such cellulose, hyaluronic acid, alginic acid, chitosan, and polysaccharide derivatives have been effectively used to improve drug administration in the treatment of eye illnesses [8].
In the treatment of eye diseases, polysaccharides such as cellulose, hyaluronic acid, alginic acid, chitosan, and polysaccharide derivatives have been successfully utilized to enhance drug administration. Nevertheless, because these medications usually do not reach the retina, vitreous, or choroid, alternative preferred drug administration routes are being created in tandem with the development of novel technologies [9]. Among its difficulties are higher tear dilution, turnover rate, and bioavailability (less than 5%). These delivery methods can increase the bioavailability of nutrients and poorly absorbed drugs, improve solubility and stability, and mask flavor and odor [10].
One promising DDS that can extend the administration, enhance stability, and lessen systemic toxicity of liposoluble medications is nanostructured lipid carriers (NLC). By adding an appropriate polymer, NLC Surface can be altered to increase its bio adhesion and retention at the disease site. NLC for ocular delivery was created by combining liquid and solid lipids that ranged in size from 50 to 1000 nm and were biocompatible and stable [11]. Hydrogel with NLC added is equivalent to Nanogel. A broad-spectrum antibiotic that can be used to treat bacterial infections is Ofloxacin. 361.3675 g/mol is its molecular weight. Through its bactericidal properties, Ofloxacin inhibits the replication of bacterial DNA by binding to the DNA gyrase enzyme. Ofloxacin prevents bacterial DNA replication by unwinding one DNA double helix into two, which is made possible by this enzyme [12].
Fascinatingly, the medication has a 100-fold higher affinity for bacterial DNA gyrase than mammalian DNA gyrase. Ofloxacin is a broad-spectrum antibiotic that works against both Gram-positive and Gram-negative bacteria. Ofloxacin inhibits DNA gyrase and topoisomerase IV, two enzymes that help avoid excessive DNA supercoiling during transcription or replication [13]. These enzymes function similarly to human topoisomerase. The drug stops the cells from doing their job, which stops regular cell division. In tablet form, Ofloxacin has a bioavailability of approximately 98%. Ofloxacin is mainly eliminated by the kidneys, where, after 48 h of administration, 65-80% of an oral dose is eliminated unchanged through urine. Ofloxacin is moderately excreted in the biliary system, however it is eliminated in the faeces in levels ranging from 4–8% of the intake [14].
MATERIALS AND METHODS
Materials: Unichem Laboratories Ltd. (C-31 & 32, Industrial Area, Meerut Road, Ghaziabad: 201003, U.P., India) sent a gift sample of Ofloxacin. The suppliers of Stearic acid and Carbopol 934 P were Loba Chemie Pvt. Ltd., Mumbai, Maharashtra 400005. Locally produced Dabur castor oil was bought. Merck Specialties Private Limited, Mumbai, 400018, was the source of Tween 80. The Guru Nanak Institute of Pharmaceutical Science & Technology Laboratory, located in Kolkata 700114, prepared double-distilled water. Merck Life Science Private Limited, located in Mumbai 40007, provided methanol and triethanolamine.
Methodology
Compatibility Study of the Materials
FTIR has been selected in this investigation to investigate the drug's compatibility with other materials. To get FTIR spectra of the medicine (Ofloxacin), the components (stearic acid, castor oil, carbopol 934 P, etc.) separately, and the mixture (nanostructured lipid carrier), KBR pellets were made and scanned [15]. The spectra were obtained using a Perkin Elmer Spectrum Two FTIR spectrophotometer [16]. The sample had to be prepared by adding potassium bromide, triturating it in a glass mortar, and then adding it to the sample container. A frequency range of 4000 - 400 cm-1 was covered by the spectrum scan [17].
Experimental Design for the Preparation of Nanostructured Lipid Carrier (NLC)
Using Design-Expert Software (Version 7.1.5), the Face Centered Central Composite Design (α = 1) was chosen for the creation of a Nanostructured Lipid Carrier (NLC). Homogenization Speed (RPM) and Liquid Lipid (mL) were selected as independent variables and set at high and low values. 13 runs have been found as a result. Table 1 contains the coded values for the independent variables [9,13,17,18].
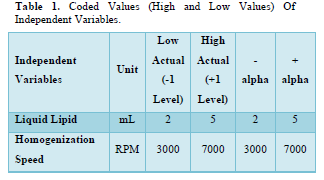
The Face-Centered Central Composite Design indicates that 13 runs were discovered and are listed in Table 2.
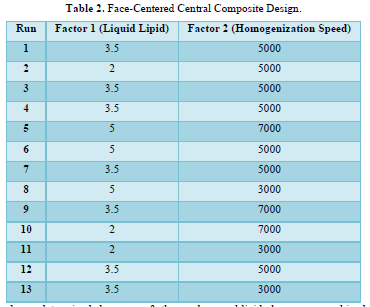
Nine runs total have been determined because of the discovery that five of the thirteen runs are duplicates.
Method of Preparation of Nanostructured Lipid Carrier (NLC)
With the aid of a magnetic stirrer (REMI 1MLH) from Remi Elektrotechnik Ltd. (Vasai, India), the lipid mixture was created by continuously swirling stearic acid, a solid lipid, and castor oil, a liquid lipid, in a heated environment between 60 and 70 degrees Celsius. Ofloxacin's aqueous phase and lipid phase were combined while heated to 60–70 degrees Celsius and with Tween 80 as a surfactant. The liquid, which was still in a beaker, was homogenized for 15 to 20 min at 3000-7000 rpm using a REMI Motor from Remi Elektrotechnik Ltd. in Vasai, India [12,19]. It was maintained at a high temperature throughout. To further homogenize the mixture, it was moved to the homogenizer tube. Table 3 provides information on the makeup of various formulations [20] (Figure 1).
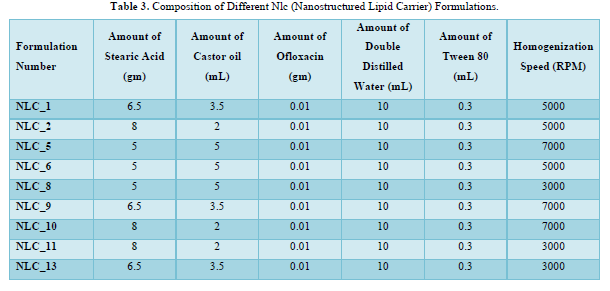
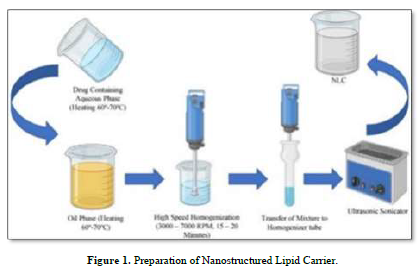
Method of Preparation of Nanogel
The synthesized NLC (about 10% W/V) was mixed with 1.25% (W/V) of hydrogel made from Carbopol 934 P dissolved in DDW. Using a magnetic stirrer, it was combined by continuously stirring. To stabilize the formulation, 0.15 milliliters of Tween 80 was added. To achieve the right consistency for the product, 1-2 drops of triethanolamine were added to the mass at the end [8,11,12].
Evaluation of Ofloxacin-loaded Nanogel
Physical Appearance
Visual observations were used to assess the homogeneity and physical appearance of the nanogel compositions [21].
pH Determination
The pH of the nanogel compositions was measured with a pH meter [22].
Determination of Particle size
Zetasizer (Version 7.11) (Malvern Instruments Ltd, UK) was used to measure the particle size of the NLC formulations at 25°C using dynamic light scattering (DLS). After dissolving in methanol and diluting the NLC formulations 100 times, the particle size and polydispersity index were determined [23].
SEM Analysis
Particle size and shape were verified using scanning electron microscopy. Following the lyophilization of the NLC-loaded hydrogel, the material was examined in a SEM (Zeiss Evo 18, Carl Zeiss Microscopy, Penta Fet X 3). The particles contained in the nanogel have a diameter of between 100 and 200 nm [24].
Spreadability Coefficient
The spreadability determinant device was used to calculate the spreadability coefficient. It is made up of a wooden block with one end attached to a pulley. The spreadability coefficient was calculated using the nanogel's properties of drag and slip. One side of the glass was attached to the wooden block. A small amount of nanogel was applied to the glass slide and moved between the two slides. Twenty grams of weight were measured and put into the pan that was fastened to the pulley. The amount of time (measured in seconds) that the slide needed to travel 5 cm was recorded. A greater spreadability coefficient is indicated by a shorter interval [25].
Determination of % Entrapment Efficiency
Using a cooling centrifuge from REMI Elektrotechnik Ltd., Vasai, India, 1 gram of drug-loaded Nanostructured Lipid Carrier (NLC) was combined with 5 mL of methanol and cold centrifuged (5°-8°C) at 12,000 RPM for 30 min. Following centrifugation, filter paper (Mesh No.: 11 µm) was used to filter the supernatant fluid. The material that had been filtered was put into a petri dish and let to dry. Following drying, 10 mL of pH 7.4 phosphate buffer was added to the petri dish and stirred using a glass rod [26].
To determine the concentration of the medication that had been entrapped, the material was then once again filtered and subjected to spectrophotometric analysis at 294 nm using a UV Visible Spectrophotometer (Jasco V-630 Spectrophotometer). For the blank NLC, whose value was deducted from the earlier outcome, the same procedure was used. The percentage of EE was calculated using the formula below [27].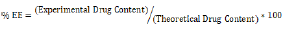
Optimization of the Formulation
The formulations were optimized using Design - Expert Software (Version 7.1.5) in terms of responsiveness, including particle size, spreadability, and % EE. Initially, the model was examined using the R-squared test and ANOVA. Next, from the model graph, the perturbation plot and the 3D response curve were derived. The software then completed the graphical and numerical optimization [12].
In-vitro Drug Diffusion Study using Dialysis Membrane
Using a Franz Diffusion Cell apparatus with a receptor compartment capacity of 45 ml and a cross-sectional area of 0.785 cm2, it was carried out through the dialysis membrane 50 (Hi-Media). The membrane was covered with the manufactured Nanogel. After then, it was fixed at the donor area. in order for the membrane to face the receiving compartment [28].
The receiver chamber was filled with Phosphate Buffer, pH 7.4. The receptor solution was constantly stirred with magnetic beads, and the temperature was maintained at 32 ± 0.5°C. The medicine was examined after the samples were taken out at various times [29].
After taking out 5 milliliters of the receptor solution, the same volume of brand-new buffer (pH 7.4) was added. The samples were examined in a UV Visible Spectrophotometer set to 294 nm for drug content analysis. Equation y = 0.032x yields the slope, and the regression coefficient is 0.9987 [16].
Permeation Study using Goat Skin
Preparation of Goat Skin
An intact goat's eye skin was procured from a butcher shop and employed in the penetration test. For an hour, it was hydrated in Phosphate Buffer (pH 7.4) [30].
Ex-vivo Permeation Study
The 45 ml Franz Diffusion cell was used for the ex vivo skin penetration investigation.
On the goat's ocular skin, 1 gram of nanogel was applied with the donor compartment facing up. Next, the skin was positioned on the diffusion cell's receiver compartment, which held phosphate buffer with a pH of 7.4. The receptor solution was continuously stirred with magnetic beads to maintain a temperature of 32 ± 0.5°C. The medicine was examined after the samples were taken out at various intervals. After removing 5 milliliters of the receptor solution, the same volume of brand-new buffer (pH 7.4) was added. At 294 nm, the samples were examined in a UV Visible Spectrophotometer to determine their drug content [31].
RESULTS
Compatibility Study of the Materials
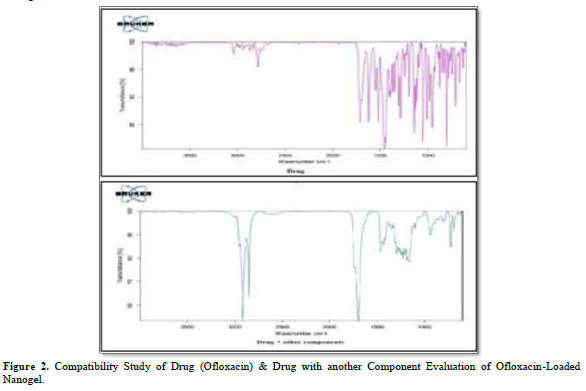
One of the crucial factors, drug-excipient interaction, is researched prior to formulation development. Separately, Ofloxacin, the drug's excipients, and IR-grade KBr were combined in a ratio of 1:100. The resulting pellets were then created by pressing 5.5 metric tons of pressure in a hydraulic press.
Stearic acid, castor oil, Tween 80, and Carbopol 934 P were the excipients. Using an FTIR spectrum analyzer, the pellets were examined across a wave number range of 4000-400 cm-1. Additionally, there aren't many differences between the peaks in the spectra of individual compounds and those of the mixture.
The mixture's spectra (1065 cm-1 for R-F where C - F Stretch is present, 2879 cm-1 for RCOOH where dimer OH is present, etc.) showed the exclusive peaks of the drug Ofloxacin (1079 cm-1 for R-F where C - F Stretch is present, 1758 cm-1 for RCOOH where monomer C = O is present, 2819 cm-1 for RCOOH where dimer OH is present, etc.). It is possible to conclude from a thorough analysis of the data that this combination can be used to move forward with nanogel formulations (Figure 2).
Physical Appearance
Based on the homogeneity, texture, and state of the products, it has been determined that NLC_1, NLC_9, and NLC_13 are better suited for creating nanogel than the other goods (Nanostructured Lipid Carriers).
pH Determination
The pH values of the nanogel formulations were determined to fall between 6.98 and 7.57. The pH values of the loaded gels in Formulation 1 (NLC_1), Formulation 2 (NLC_2), and Formulation 5 (NLC_5) were determined to be 7.43, 7.39, and 7.57, respectively. The pH values of the loaded gels in Formulation 6 (NLC_6), Formulation 8 (NLC_8), Formulation 9 (NLC_9), Formulation 10 (NLC_10), Formulation 11 (NLC_11) and Formulation 13 (NLC_13) were as follows: pH of Formulation 6 was 6.98, pH of Formulation 8 was 7.32, pH of Formulation 9 was 7.04, pH of Formulation 10 was 7.05, and pH of Formulation 13 (NLC_13) was 7.15.
Determination of Particle size
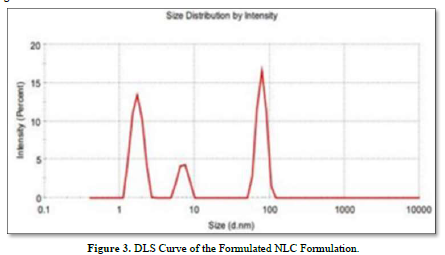
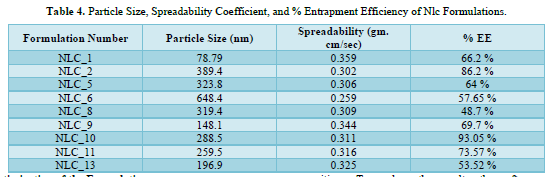
NLC Formulations were prepared using Face Centered Central Composite Design (α = 1) and Design-Expert Software (Version 7.1.5). Liquid Lipid (mL) and Homogenization Speed (RPM) were chosen as independent variables in this design, and their values were set at high and low. Liquid lipid had two low levels (in milliliters) and five high levels (in milliliters). Conversely, the homogenization speed (RPM) low level was 3000.00, and the homogenization speed (RPM) high level was 7000.00. Thirteen formulations have been found in this manner. The formulations' particle sizes ranged from 78.79 nm to 648.4 nm.
It was discovered that the particle size in Formulation 1(NLC_1) was 78.79 nm. The particle size for Formulation 6(NLC_6) was determined to be 648.4 nm. It was noted that among the nine formulations derived from Face Centered Central Composite Design, NLC_1, NLC_9, and NLC_13 had a particle size of less than 200 nm, while the remaining formulations displayed a particle size ranging from 259.5 to 648.4 nm.
The DLS curve for the prepared NLC preparation is displayed in Figure 3.
SEM Analysis
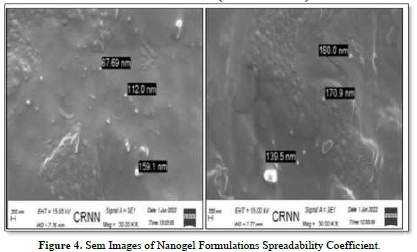
The SEM images of the nanogel formulations are displayed in Figure 4. The image displays a significant amount of particles that illustrate how NLC is distributed throughout the hydrogel to create the right nanogel. It demonstrates unequivocally that the particles fall into the nano range (67.69 to 170.9 nm).
It has been determined what the spreadability coefficient (gram. cm/sec) is for various Gel formulations that contain NLC. It was discovered that the nanogel formulations' spreadability coefficients ranged from 0.259 to 0.359 gm. cm/sec. The spreadability coefficient in Formulation 1(NLC_1) loaded gel formulation was determined to be 0.359 gm. cm/sec. The spreadability coefficient in Formulation 6(NLC_6) loaded gel formulation was determined to be 0.259 gm. cm/sec. Particle size has been discovered to be inversely related to the nanogel formulation's spreadability coefficient. The spreadability coefficient decreases with increasing particle size.
Determination of % Entrapment Efficiency
The NLC formulations'% Entrapment Efficiency was determined to be between 48.7% and 93.05%. The entrapment efficiency in Formulation 8(NLC_8) was discovered to be 48.7%. The entrapment efficiency in Formulation 10(NLC_10) was discovered to be 93.05%. Particle size has been discovered to be directly correlated with the nanogel formulation's % Entrapment Efficiency (% EE). The percentage of entrapment efficiency increases with increasing particle size. The NLC Formulations' particle size, spreadability coefficient, and percentage entrapment efficiency are displayed in Table 4.
Optimization of the Formulation
Using a Face-Centered Central Composite design technique, Ofloxacin loaded nanogel formulations were optimized.
In order to check for faults in the findings, the design exhibited 13 formulation compositions with five common compositions. To analyze the results, the software was updated to include information on particle size, spreadability, and % Entrapment Efficiency. For the purpose of evaluating the impact of independent factors on dependent components, the software produced contour graphs, polynomial equations, 3D response surface graphs, and anticipated values.
The result was found to be closer to the expected value when the expected value generated by the software was compared to the actual particle size and entrapment efficiency.
The quadratic model with the highest correlation coefficient (R2) best fits all of the responses. The quadratic response was the best option for optimization since the selected independent variable affected the dependent variables both singly and collectively. The expected R2 and the adjusted R2 were found to be in good agreement for both replies. The ANOVA result was deemed significant when the F value showed a value greater than 4. Tables 5 & 6 mentions the ANOVA of the quadratic model for the generated Ofloxacin-loaded nanogel's responses. A calculation of the software's accuracy as a percentage prediction was done.
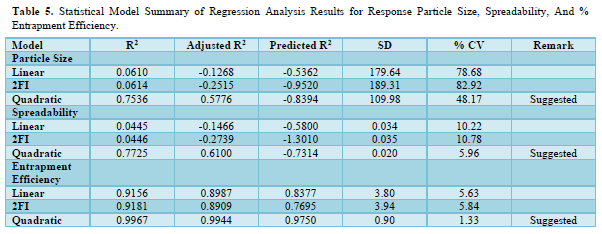
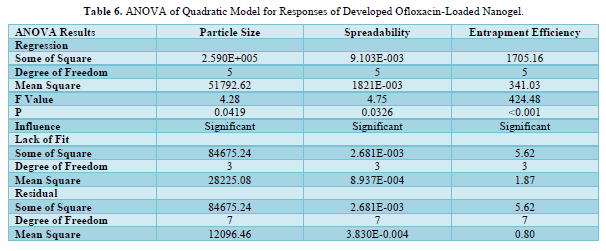
The interaction of independent factors with dependent variables is encouraged by the polynomial equation's positive sign, whereas the reverse relationship is shown by its negative value. Due to the tested independent variables total combined desirability being closer to unity, it was determined to be appropriate for optimization.
Effect of Independent Variables on Particle Size
The polynomial equation (1) and the 3D response plot in Figure 5 illustrate how the following independent variables affect particle size. The range of particle sizes for multiple batches was found to be between 78.79 (NLC_1) and 648.4 nm (NLC_6). This variation in the composition of the liquid lipid and homogenization speed caused a significant difference in particle size.
 (1)
(1)
Where, A = Liquid Lipid, and B = Homogenization Speed.
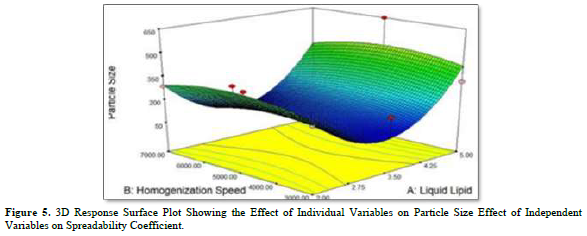
Liquid lipid harmed particle size. Therefore, to reach the minimum size, the optimal lipid content is needed. Because of the accessibility of greater concentration of liquid fat, the particle size reduces as the concentration rises. Particle size showed a favorable correlation with homogenization speed. As the homogenization cycle lengthens, the particle size grows.
Equation (1) also demonstrated how homogenization speed and liquid lipid coupled to affect particle size. Particle size showed a negative response to this combination. Therefore, it may be inferred from equation (1) that homogenization speed was not as important to particle size as liquid lipids were.
It was found that the spreadability coefficient ranged from 0.259 (based on NLC_6 gel) to 0.359 gram. cm/sec (based on NLC_1 gel) for many batches.
A notable variation in spreadability was observed as a result of the modification in the liquid lipid composition and homogenization speed.
According to the polynomial equation (2) and the 3D response plot in Figure 6, the following independent variables have an impact on the spreadability coefficient.
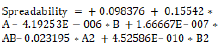 (2)
(2)
Where, A = Liquid Lipid, B = Homogenization Speed.
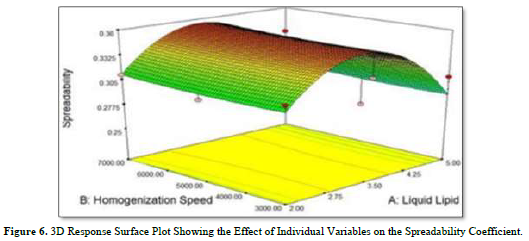
Liquid lipid had a favorable effect on spreadability. Therefore, to obtain improved spreadability, the appropriate lipid content is needed. The spreadability coefficient rises as liquid lipid concentration rises because higher concentrations of liquid lipid are more readily available. The spreadability coefficient showed a negative correlation with homogenization speed. As the homogenization cycle shortens, the spreadability coefficient rises. The combined impact of homogenization speed and liquid lipid on the spreadability coefficient was also demonstrated by equation (2). The result of this combination was an improvement in particle size.
Effect of Independent Variables on % Entrapment Efficiency (% EE)
It was found that the range of % Entrapment Efficiency for many batches was 48.7 (NLC_8) to 93.05 nm (NLC_10). There was a noticeable variance in the percentage of entrapment efficiency because of the variations in the liquid lipid composition and homogenization speed.
The polynomial equation (3) and the 3D response plot in Figure 7 demonstrate the effects of the following independent factors on the percentage entrapment efficiency.
 (3)
(3)
Where, A = Liquid Lipid, and B = Homogenization Speed.
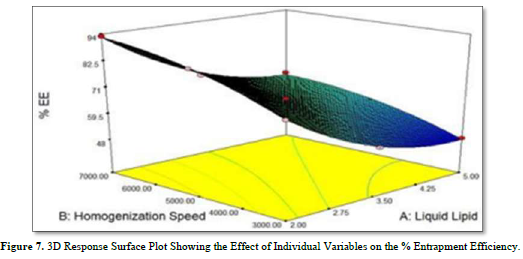
Liquid lipid had a negative impact on % EE. The percentage of EE falls when liquid lipid concentration rises because of increased liquid lipid availability. Particle size showed a favorable correlation with homogenization speed. As the homogenization speed rises, the percentage of EE rises as well.
Equation (3) also demonstrated how homogenization speed and liquid lipid coupled to affect % EE.
The percentage of EE was negatively impacted by this combination. Therefore, it may be inferred from equation (3) that homogenization speed was not as significant an influence on % EE as liquid lipids were 39 solutions have been developed by "Numerical Optimization." The desirability of each solution is 1.000. The "Solutions obtained from Numerical Optimization" are shown in Table 7.
39 optimized formulas were offered by the Design-Expert Software (Version 7.1.5). It was discovered that the formulations that had a liquid lipid quantity of 2.00 mL or less were excluded from the optimization list. These formulations have therefore been abandoned. Thirteen runs of the Face-Centered Central Composite Design model, with five formulations repeated, were produced by the Design-Expert Software.
Therefore, nine formulations were examined. Six of these 39 formulations have been added to the optimization list by the software. Formulation 1(NLC_1), which is repeated in the Numerical Optimization list, has been identified as an optimized product based on particle size. Subsequent research has been conducted on this product.
In-vitro Drug Diffusion Study using Dialysis Membrane
Figure 8 revealed the in-vitro drug diffusion profile of Ofloxacin from Formulation 1 (NLC_1). The 7 h were dedicated to the in vitro drug diffusion investigation. After 5 h, it was noted that the drug diffusion from the optimized formulation grew progressively and kept a steady pace. In 7 h, the rate of Ofloxacin diffusion was 897.79 percent.
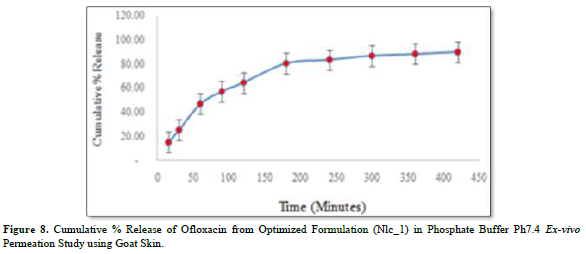
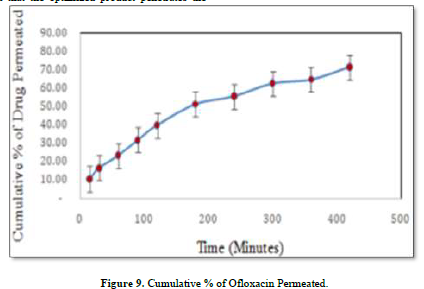
An ex-vivo experiment was carried out to verify the correlations between the in-vitro drug diffusion investigation and the dialysis membrane experiment. The outcomes were good enough to move forward. It has been decided to use healthy adult goat ocular skin for this experiment (drug permeation kinetics). The order revealed by skin permeability is as follows: mouse > rat > guinea pig > rabbit > monkey > dog > goat > ship > pig > human [32]. It has been discovered that the optimized product penetrates the goat ocular surface with Ofloxacin at a rate of around 71.33% in 7 h.
Zero-order kinetics could be used to describe drug penetration. Using P = K, the permeability coefficient was computed. Vr/S, where Vr is the receiver chamber's volume, S is the goat ocular skin's effective surface area, K is the zero-order constant, and P is the permeability coefficient of 26. The measured speed was 1.141 cm/min [33] (Figure 9).
DISCUSSION
As was previously mentioned, Ofloxacin-loaded NLC was used in this investigation. The purpose of the study was to assess the Ofloxacin-loaded nanogel's suitability for optical delivery. First, an FTIR study was used to assess the drug's compatibility with the excipients. It is possible to proceed with nanogel formulations using this combination, according to the interpretation of the FTIR spectra. Design-Expert software (Version 7.1.5) was used to develop the formulations using a face-centered Central Composite Design in order to optimize the formulation based on particle size, spreadability coefficient, and percentage entrapment efficiency. 13 formulations with 5 repetitions were offered by the software to check and analyzes errors. The Ofloxacin-loaded NLC formulations' percentage entrapment efficiency was determined to be between 48.7% and 93.05%, and their particle size measurements indicated that they ranged from 78.79 nm to 648.4 nm. Ofloxacin-loaded nanogel formulations were shown to have a spreadability between 0.259 and 0.359 gm. cm/sec. The findings were analyzed using the ANOVA. To explain the effect of Independent Variables (Factor) on the response parameter particle size, spreadability coefficient, and percentage entrapment efficiency, polynomial equations and the software's produced 3D response surface graphs were utilized. Based on the response parameter values, the software generated 39 optimal formulation predictions. Among the formulations, one with a small particle size was selected to conduct the extra investigation. Verification of the particle size distribution and surface morphology was achieved by examining the SEM photograph of the formulation. The particle size of the optimized formulation was found to be between 67.69 and 170.9 nm by performing an SEM inspection.
The ex-vivo penetration of the optimized formulation through goat optical skin and the in-vitro drug diffusion utilizing a dialysis membrane were both examined using a Franz diffusion cell. The 7 h were dedicated to the in vitro drug diffusion investigation. After 5 h, it was noted that the drug diffusion from the optimized formulation grew steadily and then stabilized. After 7 h, the Ofloxacin diffusion rate was 897.79 percent.
To verify the correlations between the in-vitro drug diffusion investigation with a dialysis membrane and the ex-vivo experiment, the former was carried out first. The findings were sufficient to move forward. The optimized solution has been discovered to have about 71.33% penetration rate of Ofloxacin through goat ocular skin in just 7 h. Zero-order kinetics could be used to describe drug penetration. Using P = K, the permeability coefficient was computed. The formula is Vr/S, where S is the goat ocular skin's effective surface area, Vr is the receiver chamber's volume, K is the zero-order constant, and P is the permeability coefficient. The measured speed was 1.141 cm/min. As a result, we may conclude from the results of this investigation that the NLC formulations effectively distribute Ofloxacin through the ocular membrane. These investigations will help to improve the formulation in the future for usage in ocular medication delivery scenarios.
- Sultana F, Manirujjaman, Imran-Ul-Haque, Arafat M, Sharmin S (2013) An overview of nanogel drug delivery system. J Appl Pharm Sci 3(8): S95-S105.
- Chauhan I, Yasir M, Verma M, Singh AP (2020) Nanostructured lipid carriers: A groundbreaking approach for transdermal drug delivery. Adv Pharm Bull 10(2): 150-165.
- Araújo J, Gonzalez E, Egea MA, Garcia ML, Souto EB (2009) Nanomedicines for ocular NSAIDs: Safety on drug delivery. Nanomedicine: Nanotechnol Biol Med 5(4): 394-401.
- Das S, Chaudhury A (2011) Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS Pharm Sci Tech 12(1): 62-76.
- Kabanov AV, Vinogradov SV (2008) Nanogels as Pharmaceutical Carriers. Published online pp: 67-80.
- Schäfer-Korting M, Mehnert W, Korting HC (2007) Lipid nanoparticles for improved topical application of drugs for skin diseases. Adv Drug Deliv Rev 59(6): 427-443.
- Azhar SNAS, Ashari SE, Zainuddin N, Hassan M (2022) Nanostructured Lipid Carriers-Hydrogels System for Drug Delivery: Nanohybrid Technology Perspective. Molecules 27(1): 289.
- Paliwal S, Kaur G (2019) Formulation and Characterization of Topical Nano Emul gel of Terbinafine. Uni J Pharm Res 3(6): 28-34.
- Riaz A, Hendricks S, Elbrink K, Guy C, Maes L, et al. (2020) Preparation and Characterization of Nanostructured Lipid Carriers for Improved Topical Drug Delivery: Evaluation in Cutaneous Leishmaniasis and Vaginal Candidiasis Animal Models. AAPS Pharm Sci Tech 21(5): 185.
- Jain K, Sood S, Gowthama rajan K (2015) Optimization of artemether-loaded NLC for intranasal delivery using central composite design. Drug Delivery 22(7): 940-954.
- Teng Z, Yu M, Ding Y, Zhang H, Shen Y, et al. (2019) Preparation and characterization of nimodipine-loaded nanostructured lipid systems for enhanced solubility and bioavailability. Int J Nanomed 14: 119-133.
- Hassan H, Adam SK, Alias E, Affandi MMRMM, Shamsuddin AF, et al. (2021) Central composite design for formulation and optimization of solid lipid nanoparticles to enhance oral bioavailability of acyclovir. Molecules 26(18): 5432.
- Sethuraman N, Shanmuganathan S, Sandhya K, Anbarasan B (2018) Design, development and characterization of nano structured lipid carrier for topical delivery of Aceclofenac. Indian J Pharm Educ Res 52(4): 581-586.
- Moghimipour E, Salimi A, Yousefvand T (2017) Preparation and evaluation of celecoxib nano emulsion for ocular drug delivery. Asian J Pharm 11(3): S543-S550.
- Abdel-Rashid RS, Helal DA, Omar MM, El Sisi AM (2019) Nanogel loaded with surfactant based nanovesicles for enhanced ocular delivery of acetazolamide. Inter J Nanomed 14: 2973-2983.
- Thakur B, Kumar I (2020) New Developed and Validated Spectroscopic Method for The Simultaneous Estimation of Terbinafine Hydrochloride and Fluconazole. Int J Pharm Pharm Sci 12(11): 19-25.
- Biswas GR, Majee SB, Roy A (2016) Combination of synthetic and natural polymers in hydrogel: An impact on drug permeation. J Appl Pharm Sci 6(11): 158-164.
- Tran TH, Ramasamy T, Truong DH, Choi HG, Yong CS, et al. (2014) Preparation and Characterization of Fenofibrate-Loaded Nanostructured Lipid Carriers for Oral Bioavailability Enhancement. AAPS Pharm Sci Tech 15(6): 1509-1515.
- Mahmood A, Rapalli VK, Gorantla S, Waghule T, Singhvi G (2022) Dermatokinetic assessment of luliconazole-loaded nanostructured lipid carriers (NLCs) for topical delivery: QbD-driven design, optimization, and in vitro and ex vivo evaluations. Drug Deliv Transl Res 12(5): 1118-1135.
- Srichaivatana K, Ounaroon A, Tiyaboonchai W (2017) Development and Characterization of Piper Retrofractum Extract Loaded Mucoadhesive Nanostructured Lipid Carriers for Topical Oral Drug Delivery. Int J Pharm Pharm Sci 9(9): 79.
- Garg J, Pathania K, Sah SP, Pawar SV (2022) Nanostructured lipid carriers: A promising drug carrier for targeting brain tumours. Future J Pharm Sci 8: 25.
- Corrias F, Lai F (2011) New Methods for Lipid Nanoparticles Preparation. Recent Patents Drug Deliv Formul 5(3): 201-213.
- Carlotti M, Pattarino F, Gasco M, Brusasca P (1993) Optimization of parameters in the emulsification process by two different methods. Int J Cosmetic Sci 15(6): 245-259.
- Moulik SP, Paul BK (1998) Structure, dynamics and transport properties of micro emulsions. Adv Colloid Interface Sci 78(2): 99-195.
- Schwarz C, Mehnert W, Lucks JS, Müller RH (1994) Solid lipid nanoparticles (SLN) for controlled drug delivery. I. Production, characterization and sterilization. J Control Rel 30(1): 83-96.
- Haider M, Abdin SM, Kamal L, Orive G (2020) Nanostructured lipid carriers for delivery of chemotherapeutics: A review. Pharmaceutics 12(3): 288.
- Amandeep, Bhatt S, Kumar M, Devi S, Saini V, et al. (2020) Recent advances in the development of the nanostructured lipid carriers for the topical fungal infections. J Rep Pharm Sci 9(2): 271-278.
- Todd PA, Faulds D (1991) Ofloxacin: A Reappraisal of its Antimicrobial Activity, Pharmacology and Therapeutic Use. Drugs 42(5): 825-876.
- Jacob S, Nair AB, Shah J, Gupta S, Boddu Sai HS, et al. (2022) Lipid Nanoparticles as a Promising Drug Delivery Carrier for Topical Ocular Therapy; An Overview on Recent Advances. Pharmaceutics 14(3): 533.
- Jain PS, Chaudhari AJ, Patel SA, Patel ZN, Patel DT (2011) Development and validation of the UV-spectrophotometric method for determination of terbinafine hydrochloride in bulk and in formulation. Pharm Method 2(3): 198-202.
- Macovei L, Gheorghe A, Schmitzer S, Burcea M, Morosanu M (2021) Ocular drug delivery systems: A review. Farmacia 69(6): 1018-1031.
- Mahdi WA, Bukhari SI, Imam SS, Alshehri S, Zafar A, et al. (2021) Formulation and optimization of butenafine-loaded topical nano lipid carrier-based gel: Characterization, irritation study, and anti-fungal activity. Pharmaceutics 13(7): 1087.
- Singh P, Jaiswal H, Lamba KA, Pahwa R, Devi S, et al. (2021) Itraconazole Loaded Nano structured lipid Carriers based In-situ Gel: Formulation, Optimization, Ex-Vivo Permeation and In-vitro Anti-Fungal Activity. Ann Rom Soc Cell Biol 25: 14216-14223.











