3743
Views & Citations2743
Likes & Shares
Extracellular matrix accumulation, basal membrane stiffness, glomerulus scarring, misplaced podocytes, mesangial cell expansion, and tubular abnormalities are the characteristics of diabetic nephropathy [3,4]. It is believed that autophagy by podocytes is an essential step in preserving kidney cells, and that impairment of this process exacerbates damage to kidney cells. Uremia, creatinine clearance rate, glomerular filtration rate (GFR), urine albumin excretion, and other parameters are altered as a result of these adjustments. End-Stage Renal Disease (ESRD) is the result of the pathophysiological changes listed above taken together [5,6].
The main causes of renal damage in diabetic kidney disease (DN) are proteinuria and hyperglycemia. Many renal cells are stimulated to release cytokines and humoral mediators in response to hyperglycemia. These drugs cause the kidney to undergo numerous alterations, including increased ECM deposition, the accumulation of AGEs, and modifications to the basal membrane's permeability, all of which exacerbate DN and encourage glomerular fibrosis [7].
According to numerous studies, oxidative stress (OS) caused by hyperglycemia plays a significant role in the development of diabetic neuropathy (DN) [8,9]. Increased OS in a hyperglycemic state activates several pathways, including the P38-MAPK, AKT, and Rheb pathways, which encourage kidney tissue fibrosis and inflammation that is related to diabetes [10,11]. Furthermore, ROS promotes TGF-β synthesis through the p38-MAPK pathway, which in turn promotes extracellular matrix (ECM) proliferation. These changes could promote inflammation and renal fibrosis, which would ultimately lead to the development of DN.
Antioxidants are chemicals that postpone or prevent cellular harm and stifle free radical processes. The antioxidant enzymes, when present with cofactors like copper, manganese, zinc, and iron, convert harmful oxidative products to hydrogen peroxide (H2O2) and finally to water [12]. According to reports, anti-oxidative therapy has demonstrated encouraging outcomes in lowering and postponing the symptoms and indicators connected to animal models of streptozotocin-induced diabetic neuropathic pain in rats [13,14].
A bioactive carotenoid called lycopene is typically present in tomatoes. The strongest antioxidant is lycopene, which is followed by lutein, α-tocopherol, α-carotene, β-crypto-xanthin, zeaxanthin-β-carotene, and β-crypto-xanthin [15].
Among other carotenoids, it is a potent oxygen quencher that may successfully reduce the production of reactive oxygen species (ROS) [16].
The only antioxidant that occurs naturally and that the body can regenerate into its active form is coenzyme Q10 (CoQ10) [17]. It is a hydrophobic material that is created internally, frequently present in mitochondria, and necessary for the oxidative phosphorylation process to produce ATP [18]. It protects tissue from oxidative damage by scavenging free radicals and inhibiting the peroxidation of protein and lipid [19].
Numerous studies have shown that, despite their disparate properties, antioxidants function more effectively and synergistically when taken together. In the cases of myocardial infarction and hepatorenal toxicity, lycopene had a synergistic benefit when combined with vitamin E and N-acetylcysteine [20,21]. When taken simultaneously, CoQ10 and curcumin had a greater migraine-causing impact [22]. The purpose of the current study was to evaluate the impact of lycopene and CoQ10 combination on DN in laboratory rats.
MATERIAL AND METHODS
Drugs and Chemicals
Zydus Cadila, India and Universal Industries, Nashik, India provided the lycopene and CoQ10, respectively. Sigma Aldrich (USA) provided STZ for purchase. The required diagnostic kits and all chemicals were standard class.
Experimental Animals
Five groups, each with six rats, were created from healthy male Wistar strain rats. The source of the rats was Wockhardt Ltd. in Aurangabad, India. The CPCSEA housing guidelines were followed. The organization's IAEC approved the study's methodology (SSDJ/IAEC/2021-22/03).
Induction of DN Using STZ
A single dose of STZ (55 mg/kg) produced in 0.2 ml of citrate buffer (0.1 M, pH 4.5) was used to create DN. A digital glucose meter (ACCU-CHEK, Roche Diabetes Care, Germany) was used to assess the blood glucose level (BGL) and confirm the diagnosis of diabetes 72 h after the STZ dosage. Rats were then observed for four weeks to see how their nephropathy was progressing.
Experimental Design
Five groups of rats were formed, with each group comprising six rats. The descriptions of the groups are as follows:
Group I: Control rat treated with vehicle alone (0.2 ml/kg/s.c.)
Group II: Rats administered with STZ (55mg/kg/s.c.) [18]
Group III: Rats treated with lycopene (5mg/kg/p.o./day) dissolved in distilled water [23]
Group IV: Rats treated with CoQ10 (10 mg/kg/p.o./day) [18]
Group V: Rats treated with lycopene (5mg/kg/p.o./day) along with CoQ10 (10mg/kg/p.o./day) simultaneously in combination. All drug treatment was given for 4 weeks.
Estimation of Biochemical Parameters in Serum and Urine
During the fourth week of the trial, rats were housed in metabolic cages individually for a single day, during which time urine samples were collected. A micro-capillary was used to draw blood from the retro-orbital nerve under ether anesthesia, and high-speed centrifugation was used to separate the serum. Samples of serum and urine were stored at -20°C and utilized for a variety of biochemical calculations. The glucometer was used to measure blood glucose. Using commercial diagnostic kits, the levels of total protein, total bilirubin, and uric acid were ascertained in urine samples, while the levels of albumin, creatinine, and urea were assessed in serum. Using the provided formula, creatinine clearance was computed [24].
Creatinine clearance (ml/min) = Urinary creatinine × (Urine volume/Serum creatinine)
Tissue homogenization
After the course of treatment, the rats were killed by beheading, the kidney was removed, and the body was cleaned with cold physiological saline. While the other kidney was utilized to estimate the antioxidant enzyme, the first kidney was preserved in 10% formalin solution for histological analysis. The kidney was thinly sliced into tiny pieces and placed in a cold 0.25M sucrose solution. Tris hydrochloride buffer (10 mM, pH -7.4), 10% w/v, was used to homogenize the renal tissues. A cooling centrifuge was used to centrifuge the homogenate for 15 min at 0°C at 10000 rpm [25]. The concentrations of nitric oxide (NO), sodium dismutase (SOD), and catalase (CAT) were measured in the supernatant, and a sediment-based technique was employed to evaluate the Na+/K+, Ca+ +, and Mg+ + ATPases.
Estimation of Oxidative Stress markers
Determination of Tissue Nitrite Level (NO) [26]
Mix 1 milliliter of homogenate with 1 milliliter of Griess reagent, then incubate at 37°C for 15 min. Measured at 40 nm against a blank for Griess reagent. Sodium nitrite was employed as the reference. Using a standard curve, the amount of nitrite in the samples was determined.
Determination of Superoxide dismutase (SOD) [27]
Chloroform was introduced after 0.5 ml of tissue homogenate had been diluted with 0.5 ml of water and 0.25 ml of cooled ethanol. After thoroughly mixing the mixture, centrifuge it. Next, 1.5 milliliters of carbonate buffer, 0.5 milliliters of EDTA solution, and 0.5 milliliters of supernatant were combined. 0.4 milliliters of epinephrine were added to start the reaction, and absorbance was measured at 480 nm in comparison to a blank. Units/mL tissue were used to express the SOD concentration. created the calibration curve using 10-125 SOD units.
Determination of Catalase (CAT) [28]
To start the reaction, combine 1 milliliter of hydrogen peroxide with 2 milliliters of diluted sample. Added 2 milliliters of the diluted sample to one milliliter of phosphate buffer (50 mM, pH 7.0) to prepare the blank. measured the absorbance at 240 nm that had dropped. The amount of catalase present in the tissue was reported as µ/ml.
Estimation of ATPase activity
Determination of Na+/K+ ATPase
One milliliter of trishydro chloride buffer, two milliliters of sodium chloride, magnesium sulfate, EDTA, potassium chloride, and ATP were added to 0.2 milliliters of homogenate. The mixture was incubated at 36°C for 15 min. After 1.0 ml of 10% TCA stopped the process, the mixture was centrifuged [29]. In order to represent the enzymatic activity, the phosphorus content of the solution was determined and expressed as nM of IP liberated/gm protein/min [30].
Determination of Ca++ ATPase
Fill a test tube with 0.1 milliliters of tris hydrochloride buffer, calcium chloride, ATP, and homogenate. For 15 min, incubate the mixture at 36°C. Add 1.0 milliliter of 10% TCA to the reaction, thoroughly mix, and centrifuge. An estimate of the supernatant's phosphorus content was made. As nM of inorganic phosphorus liberated/gm tissue /min, the enzyme activity was reported [31].
Determination of Mg++ ATPase
Fill a test tube with 0.1 milliliters of tris HCl buffer, magnesium chloride, ATP, and homogenate. For 15 min, the mixture was incubated at 36°C. A volume of 1.0 milliliter of 10% TCA was added, well mixed, and centrifuged to stop the reaction. An estimate of the supernatant's phosphorus concentration was made [32].
Estimation of Urinary Ion (Na+ & K+) In Urine by Using Flame Photometer
Sample Preparation
To assess salt and potassium, 1 to 3 milliliters of urine were diluted to 100 milliliters with distilled water. The resulting solution was then utilized directly.
Preparation of Stock Solution of Sodium and Potassium
To create a 1000 ppm solution, 2.54 g of sodium chloride and 1.9 g of potassium chloride were separately diluted in 100 ml of distilled water. Make a 100ppm solution using the above solution. In order to create 20, 40, 60, 80, and 100, prepare various concentrations of a 100ppm solution [33].
Statistical Analysis
Graph Pad Prism (version 5.0) was used to examine all variables following the implementation of ANOVA using Dunnett's multiple comparison test. The information was provided as mean ± standard error (SE) for n = 6. A statistical difference at the level of P< 0.05 was deemed statistically significant across all tested groups.
RESULTS
Effect of Lycopene, Coq10 and Their Combination on Fasting Blood Glucose Level (BGL) in STZ Induced DN Rats
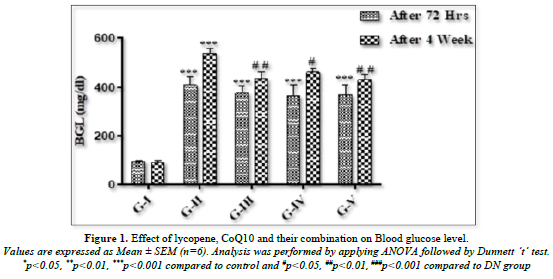
Following a 72-h injection of STZ, Figure 1 illustrates a noteworthy (p<0.001) increase in blood glucose levels in all animals as compared to the control rats. Compared to control rats, the hyperglycemia in the diabetic rats persisted until the end of the fourth week. When compared to DN animals, rats treated with lycopene (5 mg/kg p.o.) had a significant (p<0.01) drop in blood glucose. Rats receiving 10 mg/kg of CoQ10 p.o. showed a substantial (p<0.05) difference in blood glucose levels. When lycopene and CoQ10 were combined, BGL was reduced more significantly (p<0.01) in rats as opposed to DN rats.
Effect of Lycopene, Coq10 and Their Combination on Biomarkers of Kidney
All groups serum levels of urea and albumin were observed. By the end of the fourth week, there were noticeable changes in the levels of renal biomarkers including albumin and urea (Table 1). DN rats had significantly (p<0.001) higher levels of albumin and urea than normal rats because of abnormalities in the kidney's excretory function. When compared to DN rats, rats given lycopene (5 mg/kg p.o.), CoQ10 (10 mg/kg p.o.), or both (lycopene+CoQ10) exhibited a significant (p<0.01) decrease in the level of raised albumin and urea.
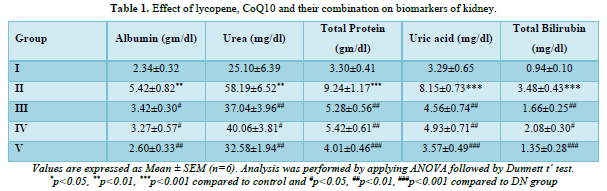
In DN rats, there were notable alterations noted in the levels of total protein, uric acid, and total bilirubin (Table 1). When DN rats were compared to normal, it was shown that their levels of total protein, uric acid, and total bilirubin were significantly (p<0.001) higher. When comparing treatment with lycopene, CoQ10, and their combination (lycopene+CoQ10) to DN rats, there was a substantial (p<0.001) drop in the level of renal biomarker.
Effect of Lycopene, Coq10 and their Combination on Creatinine Clearance
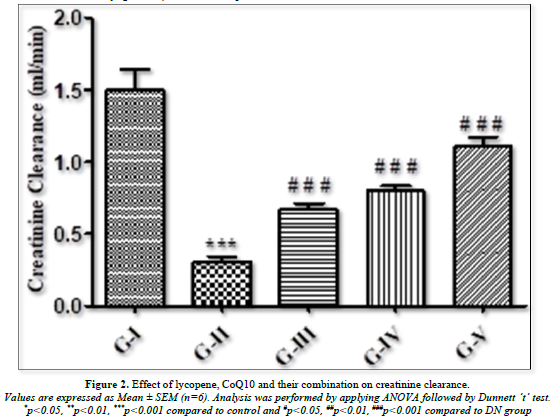
The level of creatinine clearance rate was found to have changed significantly as seen in Figure 2. When compared to normal groups, diabetic rats showed a significant (p<0.001) drop in creatinine clearance, indicating renal tissue injury. When DN rats were treated with lycopene, CoQ10, and its combination, their lowered creatinine clearance was dramatically (p<0.001) raised in comparison to normal rats.
Effect of lycopene, CoQ10 and their combination on Nitric Oxide (NO) in kidney tissue
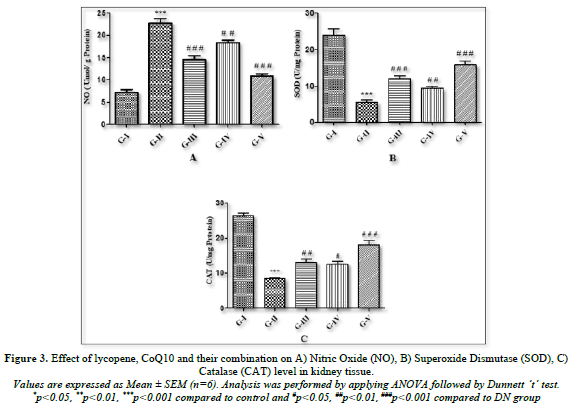
Every group's nitric oxide level was observed. Comparing the homogenate of kidney tissue from DN rats to control rats, it was found that the concentration of NO (Figure 3A) was considerably (p<0.001) higher. After a 4-week treatment, lycopene, CoQ10, and the combination of both antioxidants were given to DN rats, and the results showed a significant (p<0.001) drop in NO levels.
Effect of lycopene, CoQ10 and their combination on Superoxide Dismutase (SOD), and Catalase (CAT) level in kidney tissue
The levels of catalase and superoxide dismutase in the homogenate of renal tissue were measured in each group (Figures 3B & C). The results showed that, in comparison to the control groups, DN rats had a significant (p<0.001) decrease in SOD and CAT levels because of changes in oxidative stress markers. Rats treated with lycopene alone showed significantly (p<0.001) higher concentrations of SOD and CAT, while rats treated with CoQ10 showed slightly higher levels of the oxidative stress biomarker. On the other hand, as compared to DN groups, combined therapy of both antioxidants (lycopene + CoQ10) for 4 weeks showed a more substantial (p<0.001) rise in the level of SOD and CAT than individual medication.
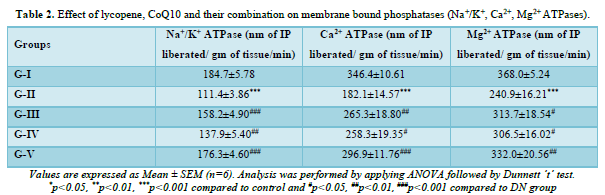
Effect of lycopene, CoQ10 and their combination on Na+/K+, Ca2+ and Mg2+ ATPases
The levels of ATPases for Na+/K+, Ca2+, and Mg2+ in the kidney homogenate were observed in each group. According to Table 2 it was observed that the concentrations of Na+/K+, Ca2+, and Mg2+ in DN rats were significantly (p<0.001) lower than in the control group. When compared to DN rats, lycopene and CoQ10-treated DN rats exhibited a significant (p<0.05) elevation in their Na+/K+ and Ca2+ levels, but a less significant increase in their Mg2+ levels. Rats given a mixture of both antioxidants for four weeks showed significantly (p<0.01) higher levels of Na+/K+, Ca2+, and Mg2+ than the DN group.
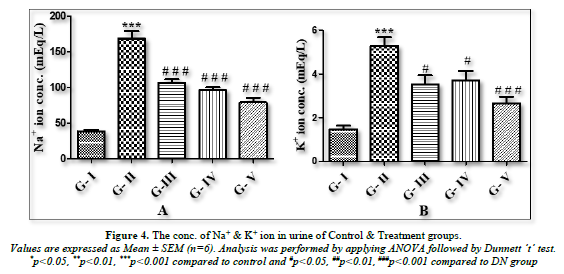
Estimation of urinary ion (Na+/ K+) in urine by Flame Photometer
The amount of urinary ion (Na+/K+) in the urine was measured using a flame photometer. Using absorbance as a guide, the unknown concentration was computed in mEq/L units. When DN groups were compared to the normal control group, there was a substantial (p<0.001) increase in the amount of urinary ions (Na+/K+) in urine. When compared to the DN rats, all treatment groups treated with lycopene, CoQ10, or a combination of the two (lycopene + CoQ10) showed a substantial (p<0.001) decrease in the amount of urinary ions (Na+/K+) in urine (Figures 4A & B).
DISCUSSION
While excessive hyperglycemia is the main cause of ROS synthesis, which is linked to the creation of oxidative damage, oxidative stress has been found to have a key role in the development and progression of DN [2,34]. Thus, the death of renal tissue, including tubular epithelial cells and podocytes in glomeruli, is caused by these free oxygen radicals produced during DN [35].
Because STZ is cytotoxic to the pancreatic beta cells that create insulin in Langerhans, injecting it subcutaneously into the experimental rats in this investigation resulted in persistent diabetes. The loss of beta cells resulted in hyperglycemia, which in turn caused oxidative stress in the renal tissue [36,37].
Antioxidants are widely used in the management of diabetes and related complications, such as diabetic foot. According to numerous assessments, lycopene is the carotenoids' most powerful antioxidant. It lowers the risk of numerous chronic diseases and is typically present in tomatoes [20,23,38,39]. Coenzyme Q10, a lipophilic substance that is frequently found in mitochondria and is crucial to the electron transport chain (ETC) during oxidative phosphorylation, is another significant antioxidant [18,40]. Because both antioxidants may scavenge free radicals, they protect cells from the oxidative damage brought on by an excess of these radicals [19,41].
A crucial baseline technique for tracking the advancement of DN is hyperglycemia [42]. In this study, elevated blood glucose levels were seen in STZ rats, which suggested significant beta cell loss. By encouraging glucose consumption by peripheral tissues or boosting insulin production by the pancreas from regenerated beta cells, the administration of lycopene and CoQ10, both alone and in combination, considerably lowers the blood glucose level [43]. They also prevent a number of hemodynamic pathways from being activated. The outcome agreed with the research that had been previously published [23].
The main symptoms of diabetic nephropathy include proteinuria, polyuria, and decreased renal function, which is indicated by an abnormal creatinine level. The increased concentration of renal biomarkers in the urine and serum of diabetic rats indicates that renal impairment is gradual and results from the kidney's decreased excretory function and regulatory role in maintaining the parameters' constant homeostasis. In the current study, the renal biomarkers total albumin and urea in serum and total protein, uric acid, and total bilirubin in urine significantly increased in the STZ-induced diabetic rats. These biomarkers indicated abnormalities in the kidney's excretory and regulatory function, which resulted in interstitial atrophy and epithelial cell necrosis from oxidative damage [44]. The last byproducts of the breakdown of proteins and purine, respectively, are urea and uric acid. Elevated urea and uric acid are caused by increased oxidative damage. Renal failure is mostly indicated by microalbuminuria and creatinine. The buildup of glucose in blood and urine, which results in the presence of protein in urine and the production of free radicals, is what caused these changes in biochemical markers in serum and urine [45].
Following the administration of lycopene and CoQ10, the biochemical parameters in the urine and serum of the DN rats showed notable alterations. These increased biomarker levels were dramatically reduced in the group treated with lycopene and CoQ10, however due to their synergistic impact, the group treated with the combination of both antioxidants reduces this level more significantly than the individual medication therapy. Because the tubular cells were damaged, the creatinine clearance was lower in DN rats. The administration of CoQ10 and lycopene as antioxidants to DN rats dramatically raises their creatinine clearance. On the other hand, their concurrent dosing increased creatinine clearance more favorably.
The delivery of STZ results in the production of free radicals and consequent oxidative damage in DN, potentially impacting the functions of endogenous antioxidant indicators. By generating free radicals, it upsets the balance between oxidants and antioxidants. The current study looked at the oxidative stress markers, NO, SOD, and CAT.
Antioxidants should be present close to the site of radical generation, competing with free radicals for the biological substrate, in order to prevent the interaction of radicals with biological molecules [46]. Oxidation of biomolecules, such as lipid peroxidation, protein damage, oxidation of single DNA and RNA nucleotides, enzyme inhibition, and apoptosis activation, will happen if an antioxidant is not present in adequate quantity to neutralize ROS [47]. Increased serum glucose levels and inos stimulation may be the cause of elevated NO levels, which are caused by endothelial cell activation. DN rats were shown to have higher NO levels in a recent investigation [48]. It was discovered that the STZ rats showed a significant decrease in LPO and NO level as compared to the DN groups following a 4-week treatment of lycopene, CoQ10, and their simultaneous dose.
The main enzyme in the antioxidant defense mechanism is SOD. By utilizing metal-catalyzed processes to convert superoxide radical anion (O2−) into H2O2, SOD performs a crucial role in safeguarding cells against ROS [49]. Cellular resistance to oxidative stress generation depends on the availability of SOD [50]. In the current investigation, OS caused by hyperglycemia resulted in a significant drop in SOD levels in DN rats. SOD levels were considerably raised by lycopene alone, while CoQ10 also raised SOD levels, though not to the same extent. However, compared to the medication alone, their combination demonstrated a more noticeable increase in SOD levels. The body spontaneously produces the heme-containing protein catalase (CAT) when it is exposed to oxygen. With the special capacity to use Fe as a cofactor to transform two H2O2 molecules into water and molecular oxygen, catalases are incredibly effective at removing H2O2 [51]. By detoxifying the produced H2O2, CAT shields cells from harm and is crucial in helping cells develop a tolerance to oxidative stress as an adaptive response [52]. DN rats were found to have significantly lower levels of GSH and catalase than control rats in the current study. Lycopene was more effective than CoQ10 at increasing GSH and CAT activity, but the combination of the two antioxidants increased the amount of reduced GSH and CAT more advantageously than either treatment alone.
The structural modification of the cell membrane in STZ diabetic rats resulted in a considerable decrease in the activities of the membrane-bound ATPases (Na+/K+ ATPase, Ca²+ATPase, and Mg2+ ATPase) as compared to control rats [53]. Greater catalytic activity of the related enzymatic reaction is frequently made possible by membrane-binding. It was observed that by preserving ionic homeostasis in the kidney, therapy with lycopene, CoQ10, and their combination enhanced the activity of membrane-bound enzymes (Na+/K+ ATPase, Ca²+ATPase, and Mg2+ ATPase). The combined effect of both antioxidants was higher than that of either medication alone.
Na+ and K+ urinary ions were estimated in urine samples from the treated and control groups in the current investigation. The primary extracellular cation, sodium (Na+), affects how bodily fluids are distributed. Potassium (K+), which is essential for healthy cell activity, is another significant ion found in urine. The body's sodium-potassium pump, or Na+-K+ Pump, depends on the ions Na+ and K+. In this study, the DN group's urine had considerably higher levels of urinary ions (Na+ & K+) than the normal control group. Renal damage brought on by ongoing hyperglycemia may be the cause of this [54]. Elevated potassium and sodium excretion in the urine is linked to a higher chance of CKD progression [55]. Urine salt and potassium excretion levels were significantly lower in the CoQ10 and lycopene therapy groups.
CONCLUSION
The current study indicates that lycopene and CoQ10, two naturally occurring antioxidants, may be able to control hyperglycemia and oxidative stress brought on by diabetes mellitus. When administered concurrently, lycopene and CoQ10 showed a notable reno protective impact by enhancing the renal antioxidant system's performance and modifying morphological alterations. Both antioxidants together proved to be more beneficial than either medication alone. The results of this study showed that the combined action of CoQ10 and lycopene was caused by their potent antioxidant properties, which inhibit the progression of DN.
ACKNOWLEDGMENTS
All the authors were thankful to the suppliers for providing drug samples as well as college authorities for their support of this research.
CONFLICTS OF INTEREST
There are no conflicts of interest.
- Marcovecchio ML, Dalton RN, Prevost AT, Acerini CL, Barrett TG, et al. (2009) Prevalence of abnormal lipid profiles and the relationship with the development of microalbuminuria in adolescents with type 1 diabetes. Diabetes Care 32(4): 658-663.
- Forbes JM, Coughlan MT, Cooper ME (2008) Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes 57(6): 1446-1454.
- Vora JP, Ibrahim HAA (2003) Clinical manifestations and natural history of diabetic nephropathy. In J. Feehally (Ed.), Comprehensive clinical nephrology pp: 425-437.
- Arora MK, Singh UK (2013) Molecular mechanisms in the pathogenesis of diabetic nephropathy: An update. Vascul Pharmacol 58(4): 259-271.
- Ritz E, Zeng XX, Rychlík I (2011) Clinical manifestation and natural history of diabetic nephropathy. Contrib Nephrol 170: 19-27.
- Garud MS, Kulkarni YA (2014) Hyperglycemia to nephropathy via transforming growth factor beta. Curr Diabetes Rev 10(3): 182-189.
- Schena FP, Gesualdo L (2005) Pathogenetic mechanisms of diabetic nephropathy. J Am Soc Nephrol 16(Suppl 1): S30-S33.
- Ighodaro OM (2018) Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed Pharmacother 108: 656-662.
- Kashihara N, Haruna Y, Kondeti VK, Kanwar YS (2010) Oxidative stress in diabetic nephropathy. Curr Med Chem 17(34): 4256-4269.
- Fakhruddin S, Alanazi W, Jackson KE (2017) Diabetes-induced reactive oxygen species: Mechanism of their generation and role in renal injury. J Diabetes Res 2017: 8379327.
- Bhattacharjee N, Barma S, Konwar N, Dewanjee S, Manna P (2016) Mechanistic insight of diabetic nephropathy and its pharmacotherapeutic targets: An update. Eur J Pharmacol 791: 8-24.
- Nimse SB, Pal D (2015) Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv 5: 27986-28006.
- Mahmoodnia L, Aghadavod E (2017) An update on diabetic kidney disease, oxidative stress and antioxidant agents. J Renal Inj Prev 6(2): 153-157.
- Tavafi M (2013) Diabetic nephropathy and antioxidants. J Nephropathol 2(1): 20-27.
- Heber D, Lu QY (2002) Overview of mechanisms of action of lycopene. Exp Biol Med 227(10): 920-923.
- Zhu R, Chen B, Bai Y, Miao T, Rui L, et al. (2020) Lycopene in protection against obesity and diabetes: A mechanistic review. Pharmacol Res 159: 104966.
- Fuke C, Krikorian SA, Couri RR (2002) Coenzyme -Q10 he. J Modern Pharm 2002: 10-20.
- Mahajan MS, Upasani CD, Upaganlawar AB, Gulecha VS (2020) Renoprotective effect of co-enzyme Q10 and N-acetylcysteine on streptozotocin-induced diabetic nephropathy in rats. Int J Diabetes Clin Res 7(123): 1-12.
- Rosenfeldt FL, Haas SJ, Krum H, Hadj A, Ng K, et al. (2007) Coenzyme Q10 in the treatment of hypertension: A meta-analysis of the clinical trials. J Human Hypertens 21(4): 297-306.
- Upaganlawar A, Gandhi H, Balaraman R (2010) Effect of vitamin E alone and in combination with lycopene on biochemical and histopathological alteration in isoproterenol-induced myocardial infarction in rats. J Pharmacol Pharmacother 1(1): 24-31.
- Elsayed A, Elkomy A, Elkammar R, Youssef G, Abdo W, et al. (2011) Synergistic protective effects of lycopene and N-acetylcysteine against cisplatin-induced hepatorenal toxicity in rats. Sci Rep 11(1): 13979.
- Mohammad P, Sarraf P, Javanbakht MH (2019) The synergistic effects of curcumin and coenzyme CoQ10 supplementation in migraine prophylaxis: A randomized, placebo-controlled, double-blind trial. Neurol Res 24: 317-326.
- Pansare K, Upasani C, Upaganlawar A, Sonawane G, Patil C (2021) Preclinical study of lycopene alone and in combination with olive oil in streptozotocin-induced diabetic nephropathy. pp: 320-332.
- Lavender S, Hilton P, Jones N (1969) The measurement of glomerular filtration rate in renal disease. Lancet 294(7632): 1216-1219.
- Jain MD (2015) Silibinin, a bioactive flavanone, prevents the progression of early diabetic nephropathy in experimental type‐2 diabetic rats. Int J Green Pharm 9(2): 118-124.
- Guevara I, Iwanejko J, Dembinska-kiec A, Pankiewicz J, Wanat A, et al. (1998) Determination of nitrate in human biological material by the simple Griess reaction. Clin Chimica Acta 274: 177-188.
- Misra HP, Fridovich I (1972) The role of superoxide anion in the autooxidation of epinephrine and a simple assay of superoxide dismutase. J Biol Chem 247(10): 3170-3175.
- Luck H (1971) Methods of enzymatic analysis (Vol. 3). New York: Academic Press.
- Bonting SL (1970) Membrane ion transport. Wiley Inter Sci 1: 254-363.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265-275.
- Hjerken S, Pan H (1983) Purification and characterization of two forms of low-affinity calcium ion ATPase from erythrocyte membrane. Biochimica et Biophysica Acta 728: 281-288.
- Ohnishi T, Suzuki T, Suzuki, Ozawa K (1982) A Comparative study of plasma Mg2+ ATPase activities in normal, regenerating and malignant cells. Biochimica et Biophysica Acta 684(1): 67-74.
- Overman R, Davis AK (1974) The application of flame photometry to sodium and potassium determination in biological fluids. J Biol Chem 168(2): 641-649.
- Zhang JQ, Fang BJ, Zhang QZ, Ji XB, Chen L (2016) Reno protective effect of lidocaine on streptozotocin-induced diabetic nephropathy. Int J Clin Exp Med 9(7): 14254-14259.
- Kukner A, Colakoglu N, Ozogul C, Naziroglu M, Firat T (2009) The effects of combined vitamin C and E in streptozotocin-induced diabetic rat kidney. J Afr Stud Dev 1(2): 29-36.
- Wang S, Yang Z, Xiong F, Chen C, Chao X, et al. (2016) Betulinic acid ameliorates experimental diabetic-induced renal inflammation and fibrosis via inhibiting the activation of NF-κB signaling pathway. Mol Cell Endocrinol 434: 135-143.
- Daniel EE, Mohammed A, Tanko Y, Ahmed A (2015) Effects of lycopene on kidney antioxidant enzyme activities and functions in streptozotocin-induced diabetic Wistar rats. Cell Biol 3(1): 1-13.
- Taheri Z, Ghafari M, Amiri M (2015) Lycopene and kidney; future potential application. J Nephropharmacol 4(2): 49-51.
- Leh HE, Lee LK (2022) Lycopene: A potent antioxidant for the amelioration of type II diabetes mellitus. Molecules 27(7): 2335.
- Hodgson JM, Watts GF, Playford DA, Burke V, Croft KD (2002) Coenzyme Q10 improves blood pressure and glycaemic control: A controlled trial in subjects with type 2 diabetes. Eur J Clin Nutr 56(11): 1137-1142.
- Bayramoglu G, Bayramoglu A, Altuner Y, Uyanoglu M,Colak S (2015) The effects of lycopene on hepatic ischemia/reperfusion injury in rats. Cytotechnology 67(3): 487-491.
- Wang GG, Lu XH, Li W, Zhao X, Zhang C (2011) Protective effects of luteolin on diabetic nephropathy in STZ-induced diabetic rats. Evid Based Complement Alternat Med 2011: 323171.
- Eze S, Afodun AM, Sulaiman S (2018) Lycopene Attenuates Diabetes-Induced Oxidative Stress in Wistar Rats. J Diabetes Endocrinol 9(2): 11-19.
- Akinnuga AM, Bamidele O, Adewumi AJ (2019) Evaluation of kidney function parameters in diabetic rats following virgin coconut oil diet. Folia Medica 61(2): 249-257.
- Sangar S, Singh (2016) Effects of streptozotocin-induced Type I Diabetes Mellitus on cation contents in urinary bladder tissues of the rat. Int J Pharm Sci Res 7(2): 789-797.
- Arora A, Sairam RK, Srivastava GC (2002) Oxidative stress and antioxidative system in plants. Curr Sci 82: 1227-1238.
- Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48(12): 909-930.
- Bhokare KH, Upaganlawar AB (2016) Neuroprotective Effects of Lagerstroemia Speciosa Extract (Banaba Leaf Extract) in Streptozotocin-Induced Painful Diabetic Neuropathy in Laboratory Rats. Pharmacologia 7(1): 9-15.
- Vijaykumar K, Arumugam VA, Ramasamy M, Natesan M, Palanisamy S, et al. (2020) Hepatoprotective effects of Psidium guajava on mitochondrial enzymes and inflammatory markers in carbon tetrachloride-induced hepatotoxicity. Drug Dev Indust Pharm 46(12): 2041-2050.
- Lee KH, Cha M, Lee BH (2020) Neuroprotective effect of antioxidants in the brain. Int Mol Sci 21(19): 7152.
- Cerny M, Habanova, H, Berka M, Luklova M, Brzobohaty B (2018) Hydrogen peroxide: Its role in plant biology and crosstalk with signaling networks. Int J Mol Sci 19(9): E2812.
- Usui S, Komeima K, Lee SY, Jo YJ, Ueno S, et al. (2009) Increased expression of catalase and superoxide dismutase 2 reduces cone cell death in retinitis pigmentosa. Mol Ther 17(5): 778-786.
- Senthil S, Sridevi M, Pugalendi KV (2007) Cardioprotective effect of oleanolic acid on isoproterenol-induced myocardial ischemia in rats. Toxicol Pathol 35(3): 418-423.
- Mestry SN, Dhodi JB, Kumbhar SB, Juvekar AR (2017) Attenuation of diabetic nephropathy in streptozotocin-induced diabetic rats by Punica granatum Linn. leaves extract. J Tradit Complement Med 7(3): 273-280.
- He J, Mills KT, Appel LJ, Yang W, Chen J, et al. (2016) Urinary sodium and potassium excretion and CKD progression. J Am Soc Nephrol 27(4): 1202-1212.






