1761
Views & Citations761
Likes & Shares
Results: Between baseline and six months of treatment, E2 changed from 89.2 to 94.1 pg/mL and p-VAS from 63.7 to 45.0 mm (p=0.507) in patients receiving tocopherol N. In contrast, the respective values in patients receiving HRT were 32.9 to 77.1 pg/mL and 78.9 to 24.3 mm (p<0.001). Many patients reported suspected rheumatoid arthritis or unknown arthropathy, but the final diagnosis was tendonitis and/or osteoarthritis in over 65.2% of the cases.
Conclusion: Most menopausal women's arthropathies, including osteoarthritis and tendonitis, improve within 2-6 months with HRT.
Discussion: We narrowed the range of undifferentiated arthritis that could develop into rheumatoid arthritis. If left untreated, osteoarthritis and tendonitis might persist and become difficult to treat.
Key-Point: Most menopausal women's arthropathies, Comprising OA and tendonitis, Improved within two months of HRT.
Keywords: Hormone replacement therapy (HRT), Osteoarthritis (OA), Rheumatoid arthritis (RA), Tendonitis, undifferentiated arthritis (UA)
Joint lesions are grouped based on their progress from onset to the time of final diagnosis into 1) tendonitis and/or spring finger; 2) OA; 3) undifferentiated arthritis (UA); 4) joints with collagen disease; 5) psychotic arthralgia.
In 2018, it was reported that hormone replacement therapy (HRT) was effective for arthropathy in menopausal women [2,3], but arthropathy was not classified in those studies. Furthermore, it has recently been reported that HRT was effective for symptoms in medium-large joints. Therefore, we diagnosed OA or tenosynovitis based on the effects of HRT on medium-large joint diseases, including low back pain, in mid-age women [4].
MATERIALS AND METHODS
Patients and disease
We enrolled 285 women with joint pain, myalgia, and tendonitis associated with menopausal symptoms for at least six months during 2021-2022.
Each patient was assigned one disease name, even if neither tendonitis nor OA could be determined as the primary disease. Fourteen common variables were included based on the patient's chief complaint at the initial consultation and the doctor's final diagnosis. Other variables were classified as miscellaneous.
The average age, body mass index (BMI), and simplified menopausal index (SMI) [5] at the first visit were 52.1±5.0 years, 22.1±3.7 kg/m2, and 54.6±5.1, respectively.
Blood tests
We assessed the levels of estradiol (E2) and follicle-stimulating hormone (FSH), the female hormones. Autoimmune rheumatic diseases were assessed by measuring the rheumatoid factor (RF), anti-cyclic citrullinated peptideCCP) antibody, anti-nuclear antibody (ANA), anti-Sjogren’s syndrome-A(SS-A) antibody, anti-dense fine speckled (DFS) 70 antibody [6], and C-reactive protein (CRP). RF ≥ 15 U/mL and anti-CCP antibody ≥ 4.5 mg/mL were considered positive, and values three times or larger were noted separately. ANA ≥ 80 times and CRP ≥ 0.3 mg/dL were considered positive.
Joints
Symptoms were recorded on the first visit as pain on movement, tenderness, and joint swelling. Over the next 2-6 months, joint symptoms were examined. Fifty joints were evaluated, including the 28 listed in the American College of Rheumatology (ACR) for RA preliminary diagnostic criteria [7], and eight DIP, two CMC, ten metatarsal phalanx (MTP), and two feet joints.
X-ray examination
Hand and foot X-ray examinations were performed when the RF and/or anti-CCP antibody was positive or a seronegative RA was suspected.
Treatment
Tocopherol N 600 mg/day was administered in 36 cases during the menopausal transition period. HRT was started in postmenopausal cases. Of these, estrogen replacement therapy (ERT) was administered to 30 patients who had undergone hysterectomy, cyclic HRT to 200 who had recently reached menopause, and continuous HRT to 19 who did not want to experience bleeding [8]. Natural E2 was mostly used. Estrogen as natural 17βestradiol was administered by a patch (70%), gel (25%), or orally (5%; containing conjugated equine estrogen).
Natural progesterone and artificial dydrogesterone (DYD) were administered at about the same rates. Medroxyprogesterone acetate (MPA) was administered in a few cases. Continuous HRT was mainly a combination of estrogen and progestin, administered orally (80%) or by a patch (20%).
A tentative definition of an early stage of osteoarthritis and tendonitis
OA happens everywhere due to osteoporosis and over use, but hand OA concerns DIP or PIP or CMC, with mainly lateral tenderness. Tendonitis concerns morning stiffness (MS), tingling or numbness, imperfect fist closure, lower or upper tenderness of tendon sheath on palmar 1~4 MCP, carpal tunnel, inter cubital, the tendons of the elbow (epicondylitis), mainly due to decline of type 2 Collagen and over use. In most cases, one of the two diseases are dominant, but sometimes they are equally dominant, in which case it is considered a comorbid condition. All four of the following conditions were met:
- X-ray showed no abnormality
- More than 1 pain on motion, or tenderness or swelling on 50 joints shown above
- Efficacy of HRT shows over 30% of E2 elevation and over 30% of FSH decline, in contrast to the baseline of E2 and FSH level
- Over 30% of the patient(p)-VAS decline, in contrast to the baseline of p-VAS
Statistical analysis
IBM SPSS Statistic for Windows, Version 26.0(IBM Corp., Armonk, NY, USA) was used for statistical analysis. A one-way analysis of variance compared variables measured at baseline and after two and six months after therapy. A post-hoc test (Bonferroni) was performed if between-group differences were detected.
Informed consent
Verbal or written informed consent was obtained from all patients before performing the blood test.
RESULTS
Background of the participants
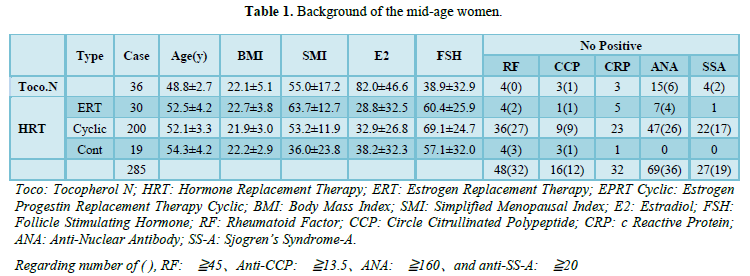
The 36 patients in the Tocopherol N (control) group had an average age of 49.2 years, E2 of 84.2 pg/mL, and FSH of 35.1 mIU/mL. The HRT group was divided into the ERT, cyclic, and continuous subgroups. Immunological tests varied among groups, but RF was positive at 15–45 units in 16 patients and ≥46 units in 32. Anti-CCP antibodies were positive at 4.5-13.4 units in 4 patients and ≥13.5 in 12. CRP was positive at ≥0.3 in 27 patients. None had CRP > 1.0, ANA was positive at ≥80 times in 69 patients and ≥160 times in 36. Among them, nine patients were positive for anti-DFS antibody, and one was associated with RA. Anti-SS-A antibodies were positive at 10-20 units in eight patients and ≥20 in 19 (Table 1).
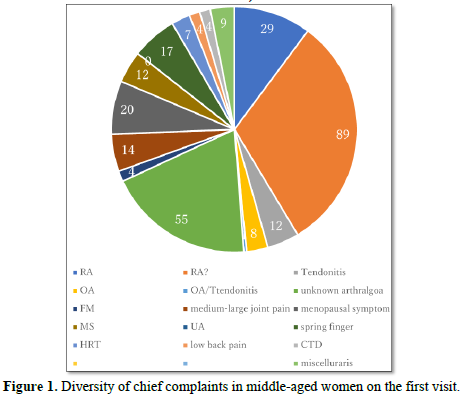
Patients’ chief complaints on the first visit
The patients' self-reports were recorded at their first visit. Twenty-nine have been diagnosed with RA at another hospital, 89 were worried about having RA, 55 had unknown arthropathy, 20 had severe menopausal symptoms rather than joint problems, 17 had overt spring finger, 14 had pain in a medium-large joint (including knee, hip, elbow, ankle, and shoulder), 12 had MS with slight joint pain, four had low back pain, and four had connective tissue disease (CTD). Nine patients had miscellaneous symptoms (Figure 1).
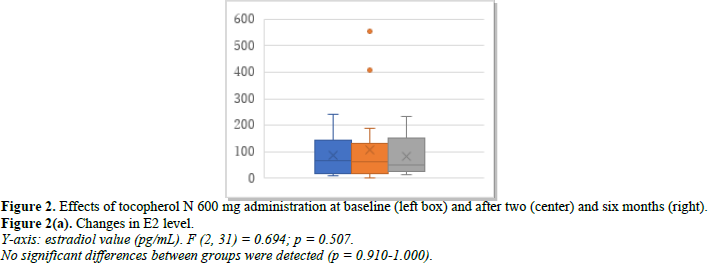
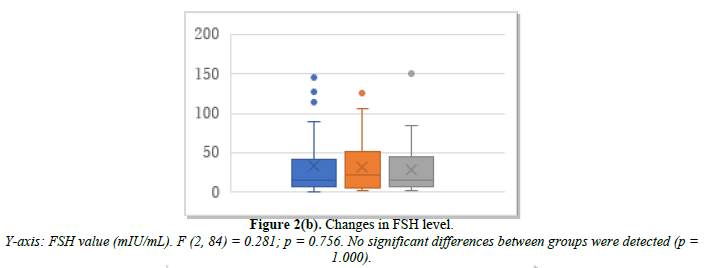
Efficacy of tocopherol N 600 mg/day as a control
The 36 patients studied included those with almost normal menstruation and those just before menopause. Changes in E2, FSH, and p-VAS between baseline and months 2 and 6 after treatment initiation are shown in Figure 2. E2 was 89.2 pg/mL at baseline, 105.8 pg/mL at month 2, and 94.1 pg/mL at month 6 (Figure 2a). FSH was 35.1 mIU/mL at baseline, 32.1 mIU/mL at month 2, and 29.6 mIU/mL at month 6 (Figure 2b). p-VAS was 63.7 mm at baseline, 47.8 mm at month 2, and 45.4 mm at month 6 (Figure 2c). Changes in joint pain are shown in Supplemental Figure 2.

Efficacy of estrogen replacement therapy
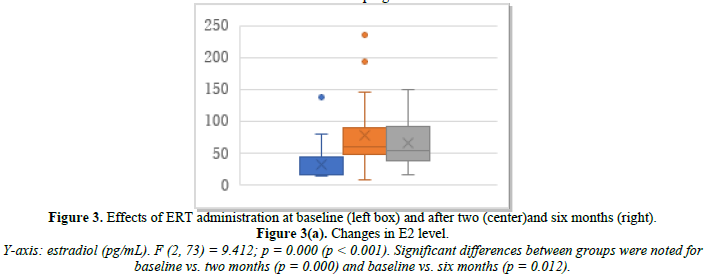
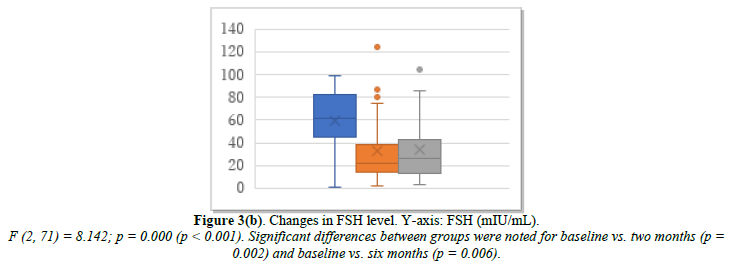
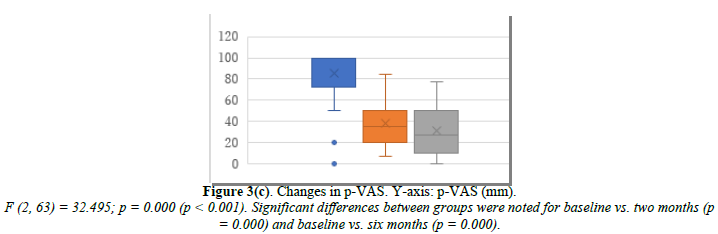
ERT was administered to 30 patients who had undergone hysterectomy. Changes in their E2, FSH, and p-VAS between baseline and two and six months after ERT initiation are shown in Figure 3. E2 increased significantly from 31.4 pg/mL at baseline to 78.1 pg/mL at month 2 and 65.7 pg/mL at month 6 (Figure 3a). FSH decreased significantly from 61.1 mIU/mL at baseline to 33.7 mIU/mL at month 2 and 35.4 mIU/mL at month 6 (Figure 3b). p-VAS decreased significantly from 85.3 mm at baseline to 37.8 mm at month 2 and 31.7 mm at month 6 (Figure 3c). Unlike the control tocopherol, ERT significantly increased E2 and decreased FSH and, correspondingly, the p-VAS score (Figure 3c). Changes in joint pain are shown in Supplemental Figure 3. The obtained data were based on estrogen effect only, without progestin (DYD) or progesterone.
Efficacy of cyclic HRT
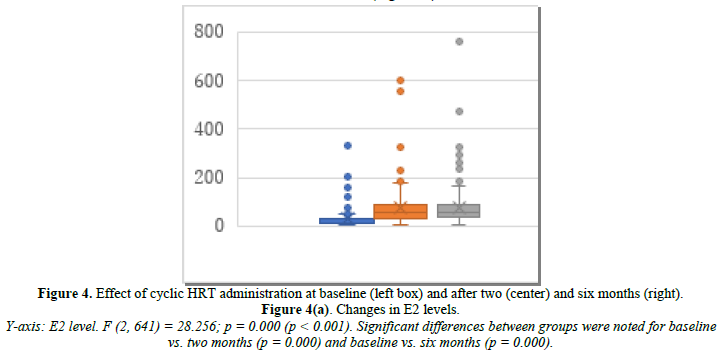
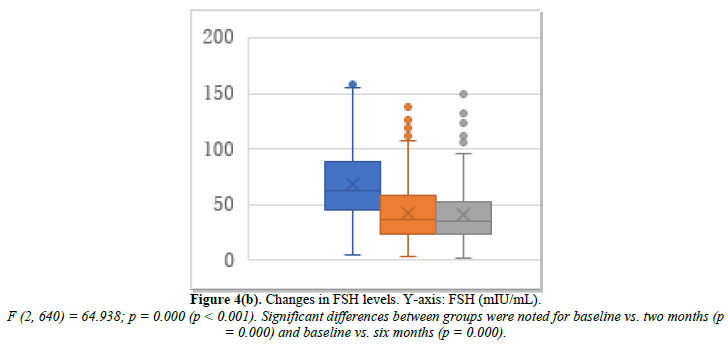
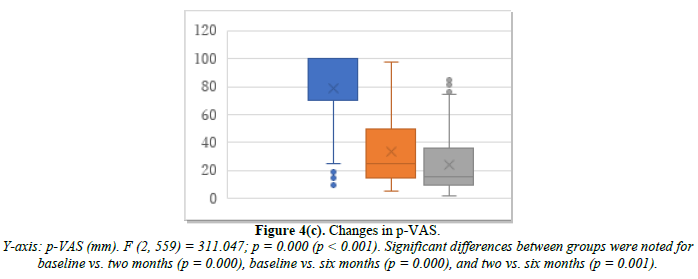
Cyclic HRT was administered to 200 patients who were close to menopause and ready to experience bleeding. Changes in E2, FSH, and p-VAS between baseline and two and six months after cyclic HRT initiation are shown in Figure 4. E2 significantly increased from 32.9 pg/mL at baseline to 76.8 pg/mL at month 2 and 77.1 pg/mL at month 6 (Figure 4a). FSH significantly decreased from 69.1 mIU/mL at baseline to 42.8 mIU/mL at month 2 and 40.9 mIU/mL at month 6 (Figure 4b). p-VAS significantly decreased from 78.8 mm at baseline to 33.4 mm at month 2 and 24.3 mm at month 6 (Figure 4c). Like ERT, cyclic HRT alleviated joint symptoms.
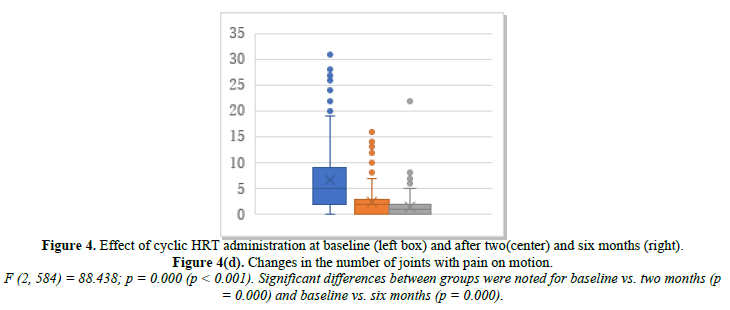
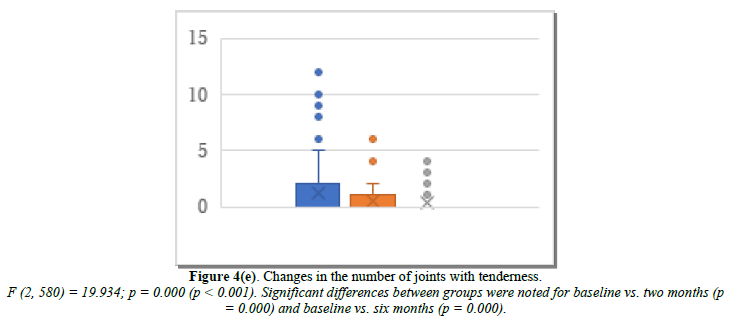
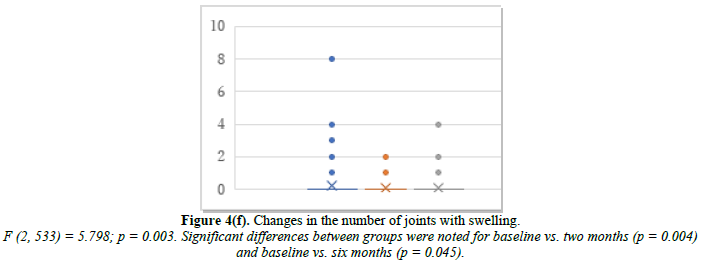
We further investigated the joint symptoms after cyclic HRT, looking at changes in the number of joints with movement pain, tenderness, and swelling. The mean number of joints with movement pain changed from 6.7 at baseline to 2.5 at month 2 and 1.4 at month 6 (Figure 4d). Joint tenderness changed from 1.22 at baseline to 0.44 at month 2 and 0.32 at month 6 (Figure 4e). Joint swelling changed from 0.26 at baseline to 0.05 at month 2 and 0.10 at month 6 (Figure 4f).
Efficacy of continuous HRT
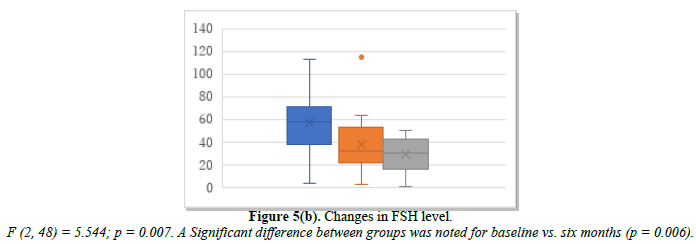
Continuous HRT was administered to 19 patients who had a history of endometriosis or adenomyosis and who requested HRT for the first time after the age of 55. Changes in E2, FSH, and p-VAS between baseline and two and six months after continuous HRT initiation are shown in Figure 5. E2 increased from 38.2 pg/mL at baseline to 40.2 pg/mL at month 2 and 61.9 pg/mL at month 6 (Figure 5a). FSH decreased from 56.9 mIU/mL at baseline to 38.2 mIU/mL at month 2 and 28.4 mIU/mL at month 6 (Figure 5b). p-VAS decreased from 78.9 mm at baseline to 25.7 mm at month 2 and 21.2 mm at month 6 (Figure 5c). Changes in joint pain are shown in Supplemental Figure 5. Like ERT, continuous daily administration of progestin or progesterone alleviated joint symptoms.

 The final diagnosis made by the doctors
The final diagnosis made by the doctors
Definitive diagnoses were made based on the patient’s self-report, the regress in joint symptoms, and blood tests after two and six months. Nine items in the patient’s chief complaint at the first consultation commonly matched the doctor's final diagnosis, while six showed the reverse association.
Of the 29 cases diagnosed with RA in another hospital, only nine were diagnosed with RA. Eighty-nine patients worried about RA due to a positive RA factor, 55 complained of joint pain of unknown cause, and 14 had pain in their medium-large joints (Figure 6). Tendonitis and osteoarthritis were the most frequently diagnosed conditions by doctors (n = 186).
 DISCUSSION
DISCUSSION
Twenty-one middle-aged women visited the clinic seeking treatment for menopause after being diagnosed with tendonitis, Heberden's nodes, or Bouchard's nodes by an orthopedic surgeon, 88 were convinced they did not have RA at their first visit, and 56 did not know what their joint symptoms meant. Complaints related to joint pain in middle-aged females are uncommon in middle-aged males. It could be assumed that the cause is related to the female hormones, but the details remain unknown [2,9]. Here, we reported the effectiveness of HRT for postmenopausal arthralgia/arthritis. As a control, tocopherol N 600 mg was administered to 36 women at the menopausal transition stage. Tocopherol N was shown to be effective for vasomotor symptoms such as palpitations, hot flashes, the plasma lipid profile, etc. [10,11], and arthralgia/arthritis, including OA and tendonitis. Tocopherol N was effective when menstruation was almost normal but ineffective when menopause was almost reached. Consequently, it was not always effective after six months in this study. Although the effect of tocopherol N for arthralgia/arthritis is derived from its anti-inflammatory activity [12], it is thought to increase the blood flow to the ovarian arteries and improve ovarian function. However, the effect is temporary, and once menopause is reached, HRT is required.
Joint symptoms during menopause are diverse, and many aspects remain unclear. There are various symptoms, such as MS, tingling in the fingers, inability to grasp or open the fingers, and pain when grasping, in addition to PIP and DIP joint pains. This study focused on motion pain, tenderness, and swelling. HRT was effective against these joint symptoms. This effect was rapid, showing noticeable improvement within two months. Although there were no untreated controls, it was not a natural course since most patients came to the clinics because their previous treatments, including Chinese herbal medicine and various supplements, had been ineffective. Once they switched to HRT, they could see marked improvement within two months.
The 2010 EULAR/ACR preliminary RA standards [13] appear very well designed for joint scoring. In particular, the progression of RA is slow when only pain in a medium-large joint is present. Findings in medium-large joints do not contribute to early RA diagnosis because the score is 0 for one location and only 1 for 2-10 locations. It is unclear why medium-large joint arthritis was not emphasized in the preliminary diagnostic criteria. Recently, HRT has become the first choice for treating large joint disease. A large-scale study on the knee reported that the prevalence of knee problems in people using HRT was significantly lower than in those who did not [14], possibly because HRT prevents osteoarthritis caused by the progression of osteoporosis [15]. On the other hand, it is difficult to differentiate PIP joint pain from early RA symptoms. Swelling and tenderness are observed in the early stages of hand OA, making distinguishing it from early RA difficult. The PIP and DIP symptoms disappear after two months of HRT, making it a good way to differentiate between hand OA and RA. Moreover, HRT prevents hand OA progression, or else hand deformity will be the natural course [16,17]. Administration of estrogen helped produce collagen in the subcutaneous layer, strengthening the cartilage at the temporomandibular joint in mice [18]. Tendonitis is the inflammation of the tendon sheaths in the wrist, fingers, and calcaneus. In a study on mice, administration of E2 suppressed inflammation of the Achilles tendon sheath [19]. Tendons and ligaments are composed mainly of fibrous collagen proteins. The pain in the fingers and tendonitis caused by aromatase inhibitors administered to treat breast cancer [20] can be alleviated with Estriol (E3) after ten years.
More importantly, of the 29 patients diagnosed with RA at their first visit, only nine were diagnosed with RA after six months of treatment at our clinics. Many patients came to see us because they felt no improvement after receiving RA treatment that included methotrexate (MTX) or biologics. Although we do not discuss the details here, some cases had UA that had not yet developed into RA, and some had UA combined with early-stage tendonitis or OA. In Colombia, half of the cases diagnosed with RA in 2015 were not diagnosed with RA when reviewed again three years later. Instead, they were diagnosed with OA or remained undiagnosed [21]. Compared to those who did not receive HRT, treated middle-aged women positive for anti-CCP antibodies had a 30% lower risk for RA progression [22]. We continue administering HRT to patients with UA positive for anti-CCP antibodies.
Finally, twenty years have passed since it was reported that HRT increases the risk of breast cancer, endometrial cancer, and thrombosis. In that report, the researchers included a high percentage of women with a smoking habit, obesity, and diabetes. Furthermore, the starting mean age was 63.5 years, and the administration method (the combination of estrogen and progestin) was not recommended [23]. Recently, a critical report stated that small problems other than these risk factors also existed [24]. However, the risk of breast cancer remained high, depending on the type of progestin used and the duration of treatment [25].
Limitations of this study include the lack of formal double-anonymized testing and the limited number of clinics that administer HRT. The effects on psychogenic or CTD arthralgia remain unclear because of the small number of patients. This is a topic for future consideration.
In summary, of the 286 participants, 21 (7%) were diagnosed with OA or tendonitis at the initial examination, and 186 (65.2%) were diagnosed with early OA and tenosynovitis six months after a treatment such as HRT was administered. Most menopausal women's arthropathies, comprising OA and tendonitis, improved within two months of HRT. By observing the treatment outcomes, we can narrow down the range of UA that could develop into RA. If left untreated, OA and tendonitis could persist and become difficult to treat.
ACKNOWLEDGMENTS
We thank Dr. Amano (pharmacist) and Dr. Yamanaka (Department of Orthopedics), Kanagawa Saiseikai Hospital, for providing critical references and advice.
SUPPLEMENTAL DATA
Supplemental data are available at J Rheumatology Res.
ETHICAL APPROVAL
This study followed the tenets of the Helsinki Declaration.
AUTHOR’S CONTRIBUTIONS
Miyachi K: Attended patients with menopause and rheumatic disease, performed data analysis, and drafted the manuscript.
Ihara A: Attended patients with menopause.
Uehara M: Attended patients with menopause.
Yamamoto K: Statical analysis.
Sasse B: English editing and discussion.
Okano H: Attended patients with menopause.
Koyama T: Overall supervision.
CONFLICTS OF INTEREST
Authors declare that they do not have any conflict of interest.
- Szoeke CEI, Cicuttini F, Guthrie J, Dennerstein L (2005) Self-reported arthritis and the menopause. Climacteric 8: 49-55.
- Miyachi K, Sasse B, Ihara A (2018) THU0695 Does hormone replacement therapy prevent undifferentiated arthritis progressing to rheumatoid arthritis? Ann Rheum Dis 77(2): 540-541.
- Miyachi K, Sasse B, Nomoto S, Igarashi T, Mashiba S, et al. (2019) The treatment of women with postmenopausal undifferentiated arthralgia - the first report of efficacy of hormone replacement therapy. Am J Biomed Sci Res 1(3): 124-129.
- Pang H, Chen S, Klyne DM, Harrich D, Ding W, et al. (2023) Low back pain and osteoarthritis pain: A perspective of estrogen. Bone Res 11(1): 42.
- Kitanohara M, Yamamoto T, Masunaga S, Ohishi M, Komatsu Y, et al. (2017) Effect of porcine placental extract on the mild menopausal symptoms of climacteric women. Climacteric 20(2): 144-150.
- Watanabe A, Kodera M, Sugiura K Usuda T, Tan EM, et al. (2004) Anti-DFS70 antibodies in 597 healthy hospital workers. Arthritis Rheum 50(3): 892-900.
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, et al. (2010) Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 62(9): 2569-2581.
- Gambacciani M, Levancini M (2015) Hormone replacement therapy: Who should be treated? Minerva Gynecol 67(3): 249-255.
- Nagamine R, Maeda T, Shuto T, Nakashima Y, Hirata G, et al. (2001) Menopausal syndrome in female patients with rheumatoid arthritis. Mod Rheumatol 11: 230.
- Feduniw S, Korczyńska L, Górski K, Zgliczyńska M, Bączkowska M, et al. (2023) The Effect of Vitamin E Supplementation in Postmenopausal Women-A Systematic Review. Nutrients 15(1): 160.
- London RS, Sundaram GS, Murphy L, Goldstein PJ (1983) The effect of alpha-tocopherol on premenstrual symptomatology: A double-blind study. J Am Coll Nutr 2(2): 115-122.
- Kiyose C, Takeuchi H, Yabe Y, Nojima T, Nagase M, et al. (2021) Effect of δ-Tocopherol on Mice Adipose Tissues and Mice Adipocytes Induced Inflammation. J Oleo Sci 70(9): 1307-1315.
- Varache S, Cornec D, Morvan J, Devauchelle-Pensec V, Berthelot J-M, et al. (2011) Diagnostic Accuracy Of ACR/EULAR 2010 Criteria for Rheumatoid Arthritis in a 2-Year Cohort. J Rheumatol 38(7): 1250-1257.
- Jung JH, Bang CH, Song GG, Kim C, Kim JH, et al. (2018) Knee osteoarthritis and menopausal hormone therapy in postmenopausal women: A nationwide cross-sectional study. Menopause 26(6): 598-602.
- Mizunuma H, Taketani Y, Ohta H, Honjo H, Gorai I, et al. (2010) Dose effects of oral estradiol on bone mineral density in Japanese women with osteoporosis. Climacteric 13: 72-83.
- Yang X, Yan K, Zhang Q Yusufu A, Ran J (2023) Meta-Analysis of Estrogen in Osteoarthritis: Clinical Status and Protective Effects. Altern Ther Health Med 29(1): 224-230.
- Burkard T, Rauch M, Spoendlin J, Prieto-Alhambra D, Jick SS, et al. (2020) Risk of hand osteoarthritis in new users of hormone replacement therapy: A nested case-control analysis. Maturitas 132: 17-23.
- Robinson JL, Cass K, Aronson R, Choi T, Xu M, et al. (2017) Sex differences in the estrogen-dependent regulation of temporomandibular joint remodeling in altered loading. Osteoarthritis Cartilage 25(4): 533-543.
- Wang F, Shan H, Song G, Chen S, Zhang C, et al. (2022) 17β-Estradiol attenuates inflammation and tendon degeneration in a rat model of Achilles tendinitis. Immunopharmacol Immunotoxicol 44(4): 556-564.
- Regent-Smith AJ, Childers EJ, Dzwierzynski WW, Morgan AL (2023) Incidence and Treatment Efficacy of Trigger Finger in the Breast Cancer Population on Aromatase Inhibitors. Hand (N Y) 18(2): 250-253.
- Gomez D, Saavedra-Martinez G, Villarreal L (2015) Misdiagnosis of rheumatoid arthritis-The photography. Ann Rheuma Dis 74 Suppl 2: 689.
- Orellana C, Saevarsdottir S, Klareskog L, Karlson EW, Alfredsson L, et al. (2015) Postmenopausal hormone therapy and the risk of rheumatoid arthritis: Results from the Swedish EIRA population-based case-control study Eur J Epidemiol 30(5): 449-457.
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, et al. (2002) Writing group for the WHI investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA 288(3): 321-333.
- Bluming AZ, Hodis HN, Langer RD (2023) Tis but a scratch: A critical review of the Women's Health Initiative evidence associating menopausal hormone therapy with the risk of breast cancer. Menopause 30(12): 1241-1245.
- Vinogradova Y, Coupland C, Hippisley-Cox J (2020) Use of hormone replacement therapy and risk of breast cancer: Nested case-control studies using the QResearch and CPRD databases. BMJ 371: m3873.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)
- Journal of Infectious Diseases and Research (ISSN: 2688-6537)
- International Journal of Internal Medicine and Geriatrics (ISSN: 2689-7687)
- Journal of Oral Health and Dentistry (ISSN: 2638-499X)
- Journal of Pathology and Toxicology Research
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)
- Journal of Psychiatry and Psychology Research (ISSN:2640-6136)



















