135
Views & Citations10
Likes & Shares
Materials and Methods: A total of 178 pregnant women were recruited in the first trimester and those with TSH between 0.1-2.4 mIU/L were considered as euthyroid and 2.5-4mIU /L were labelled as SCH. Women with SCH underwent testing for TPOAb. All women were followed until delivery and fetomaternal outcomes were assessed.
Results: Among SCH group, there was a significantly higher proportion of overweight & obese women (76/91 (83.51%) vs 59/87 (68%), p = 0.031). The neonatal intensive care unit (NICU) admission was higher with adjusted odds ratio of 3.24 (1.41-7.43) in women with SCH as compared to euthyroid women. Otherwise, there was no difference in fetomaternal outcomes between the two groups. The proportion of gestational diabetes mellitus, intrauterine growth retardation, and still birth was higher in SCH women with TPOAb as compared to euthyroid women.
Conclusions: There appears to be no difference in pregnancy outcomes between women with SCH and euthyroid women except higher NICU admission in SCH group. Future multi center large prospective studies are required to understand better about the pregnancy outcomes in these women.
Keywords: Euthyroid, Hypothyroid, Maternal, Perinatal, Subclinical
Few studies [6-9] showed that SCH (TSH between 2.5-4 mIU/L) was associated with several obstetric complications, including miscarriage, gestational hypertension (HTN), pre-eclampsia, placental abruption, gestational diabetes mellitus (GDM), intrauterine growth restriction (IUGR), preterm birth, and low birth weight (LBW) whereas others [10-13] did not reveal it in comparison to euthyroid (TSH between 0.1-2.4 mIU/L) mothers. These conflicting results might be due to variability in timing of TSH measurements, assessment of TPO Ab status and presence of other confounding factors in different studies [14]. The adjusted odds of adverse pregnancy outcomes were lower in treated women than in untreated women if their pre-treatment TSH concentration was 4.1-10 mIU/L but not if it was 2.5-4.0 mIU/L [15-18]. Rather, LT4 therapy increases the risk of poor pregnancy outcomes like preterm delivery, GDM, gestational HTN, and preeclampsia in SCH women with TSH between 2.5 and 4.0 mIU/L [19]. However, when a meta-analysis was performed using a TSH diagnostic cut-off of 4.0 mIU/L, pregnant patients with SCH had higher risk of hypertensive disorders of pregnancy both above and below this threshold compared with euthyroid pregnant women [20]. Additionally, the presence of TPO Ab also affects the fetomaternal outcomes like GDM and decreased fetal growth in SCH mothers as shown in meta-analysis by Derakshan [21] and Kent [22]. Moreover, there is a paucity of data in the Indian population [16]. And there is still insufficient evidence in the current literature whether the 2017 ATA guidelines are applicable to Indian pregnant women. With the goal of validating the current ATA guidelines among Indian subjects, this study was performed with the aim of comparing the pregnancy outcomes between SCH with and without TPO Ab and euthyroid women.
MATERIALS AND METHODS
This prospective cohort study was carried out in the department of obstetrics and gynecology of a tertiary care center in India from January 2020 to September 2021. The study protocol was registered with Clinical Trial Registry of India (Trial REF/2020/09/036436) after getting approval from the ethics committee of the institute (JIP/IEC/2019/441). The primary objective of the study was to compare the pregnancy outcome between SCH and euthyroid women. The secondary objectives were to find out the proportion of women with SCH having TPO Ab and to see the effect of TPO Ab positivity on fetomaternal outcomes. Pregnant women with TSH levels between 2.5 and 4 mIU/L were labelled as SCH and subjects with TSH levels between 0.1 and 2.4 mIU/L were considered as euthyroid. All of them had normal gestational age adjusted serum total T4 level [4]. Sample size was calculated using Open Epi software version 3.1 by considering the expected proportion of miscarriage [23] of 2.2% among euthyroid and 15.2% among women with SCH with 95% CI and power of 80%. The total sample size was estimated to be 178.
Pregnant women attending antenatal outpatient department or admitted in antenatal ward of the institute fulfilling the following inclusion and exclusion criteria were selected. The history (present and past pregnancy details including history of infertility and treatmentreceived), menstrual, personal and family history were noted in a predesigned proforma. Gestational age was calculated as per last menstrual period or early dating ultrasonographic scan depending on the reliability. General physical examination including pallor, goiter, pulse rate and blood pressure were noted. Height and weight of the study subjects were also recorded. Body mass index (BMI) was calculated by dividing the pre-pregnancy weight in kilograms by the height in meters squared. BMI between 18.5 to 22.9 kg/m2 as normal, 23 to 24.9 kg/m2 as overweight and ≥25 kg/m2 as obesity according to Asian Indian guidelines [24]. All the subjects were followed up till delivery and the feto-maternal outcomes were noted.
Hypertensive disorders of pregnancy include preeclampsia and gestational HTN. Gestational HTN is a condition with a blood pressure of more than 140/90 mm Hg in two occasions 4 hours apart with or without proteinuria after 20 weeks of gestation in a previously normotensive woman. Pre-eclampsia includes gestational HTN with proteinuria or in the absence of proteinuria a new onset thrombocytopenia, renal insufficiency, impaired liver function, pulmonary edema or headache. GDM is a condition in which carbohydrate intolerance with recognition or onset during pregnancy. Miscarriage is defined as expulsion of products of conception before the period of viability. Oligohydramnios is the condition where the amniotic fluid index is less than 5 cm in a term pregnancy. Intrauterine growth restriction (IUGR) is defined as failure of fetus to achieve its genetic growth potential. Premature rupture of membrane (PROM) is a condition where the amniotic membranes rupture before the onset of labor. Labour onset can be spontaneous or induced. Induction of labor is defined as initiation of uterine contractions after the period of viability prior to spontaneous onset of labor. Preterm delivery includes those pregnancies with delivery before 37 completed weeks of gestation. Instrumental delivery includes all the deliveries where either forceps or ventouse was used to deliver the fetal head. Cesarean section is delivery of the fetus after the period of viability through abdominal and uterine incisions.
After delivery, the neonatal birth weight, head circumference (HC) and APGAR scores (1 and 5 min) were recorded and low APGAR score was defined as < 7. Neonatal resuscitation and decision for neonatal intensive care unit (NICU) admission were taken by the neonatologist. We categorized our babies as less than 2.5 kilograms, 1.5 kilograms and 1 kilograms as low birth weight (LBW), very low birth weight (VLBW) and extremely low birth weight (ELBW) respectively. Stillbirth is defined as death fetus beyond 28 weeks of gestation. Respiratory distress syndrome (RDS) is a condition that develops due to pulmonary immaturity and surfactant deficiency. Neonatal sepsis is a clinical syndrome of systemic illness with bacteremia in the first 28 days of life. Congenital anomalies are structural defects that are present at birth. Low HC was defined as HC < 32 cm [25].
The thyroid function test (TFT) that includes serum TSH & total thyroxine (T4) and TPO Ab were done in duplication in endocrinology laboratory of the institute. Five ml. of venous blood was drawn from ante-cubital vein of the subjects and was processed by the chemiluminescent assay system (ADVIA Centaur XP Immunoassay System, Siemens Healthcare Global, USA) in accordance with the manufacturer’s instructions. The total coefficient of variation of TSH and total T4 assay was 3.17% and 5.55% respectively. The cut off to indicate positivity for TPO Ab was 60 U/mL, which was estimated only in pregnant women with SCH.
Continuous variables were represented as mean ± SD or median with inter-quartile range (IQR), depending on the variable’s distribution. The normality of the data was assessed using appropriate tests. Categorical variables were expressed as a percentage and were analyzed using Chi-squared or Fisher’s test. Independent Student’s t-test and Mann-Whitney U test were done to compare the continuous variables based on the normality. Both unadjusted and maternal BMI adjusted odds ratio (aOR) with 95% confidence intervals (95% CIs) for fetomaternal outcomes was calculated. P-value < 0.05 was taken as statistically significant. The data was analyzed using STATA 14.0.
RESULTS
The study included a total of 87 euthyroid and 91 SCH pregnant women as shown in Figure 1. The mean age of the study participants was 25.91 years and 60% of the study population were primigravida. SCH women had higher BMI compared to euthyroid subjects (27±5.2 vs. 25±4.5 kg/m2, P=0.01) as shown in Table 1. Only 14% SCH women had normal BMI in comparison to 30% among euthyroid antenatal mothers (P=0.03). Only 14 (15.38%) pregnant women with SCH had positive TPO Ab.
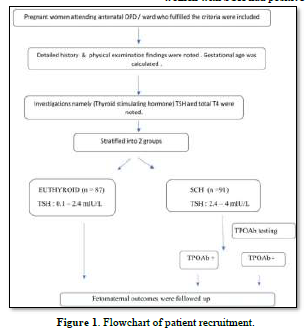
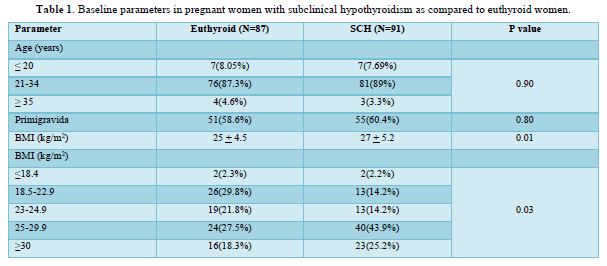
Table 2 shows the comparison of maternal outcomes between pregnant women with SCH and euthyroid mothers. There was no overall difference in maternal outcomes between euthyroid and SCH women. The need for induced labor was twice more common among pregnant mothers with TPO Ab -ve SCH compared to euthyroid subjects as shown in Table 3. However, TPO Ab positive SCH women have higher risk of both GDM (aOR: 3.92 (1.17-13.08)) and IUGR (aOR: 4.79 (1.48-15.56)) compared to euthyroid mothers as shown in Table 4.
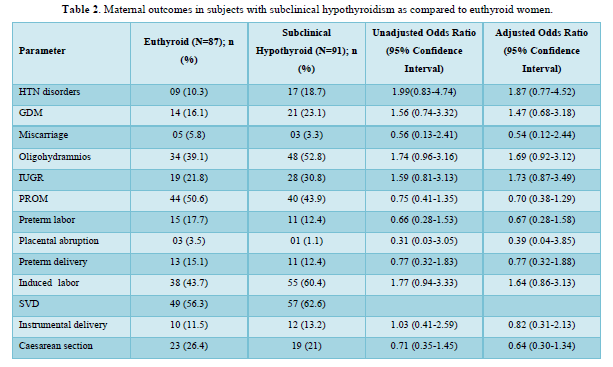
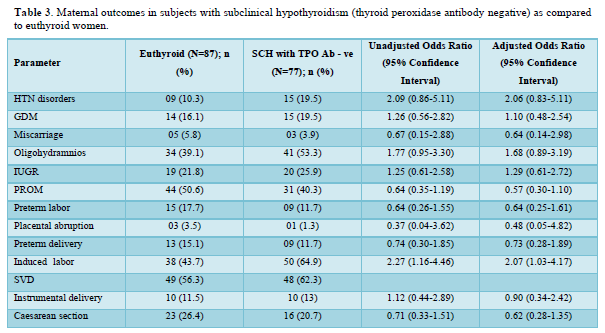
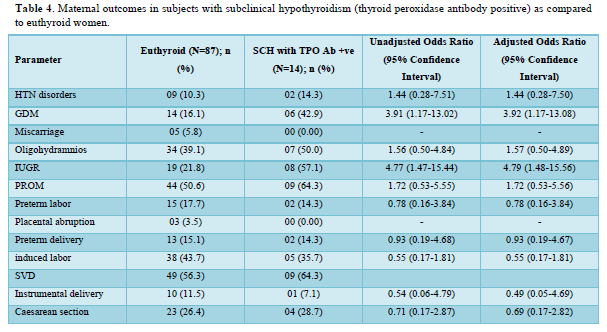
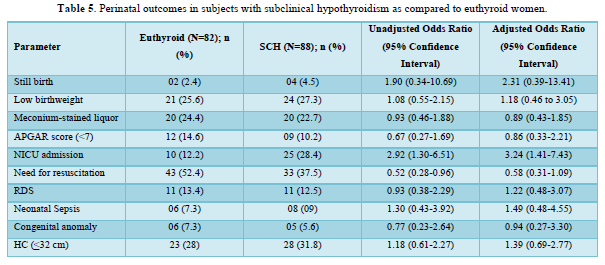
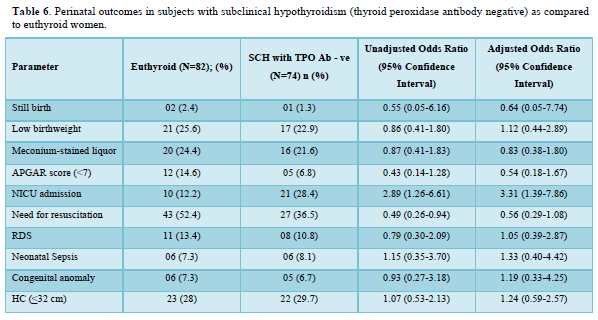
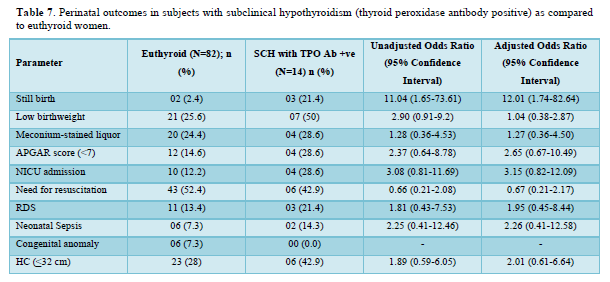
Table 5 shows the comparison of perinatal outcomes between pregnant women with SCH and euthyroid mothers. NICU admission was more in women with SCH as compared to euthyroid mothers with aOR of 3.24 (1.41-7.43). Similar result was also found among TPO Ab -ve SCH mothers as shown in Table 6. Out of 21 neonates with LBW in euthyroid group, 3 and 4 babies were VLBW and ELBW respectively. Similarly, out of 24 neonates with LBW in SCH group, 3 and 2 babies were VLBW and ELBW respectively. No neonate had birth weight > 4kg in our study. There were 2 stillbirths in the euthyroid group out of which one fetus had anencephaly and the other fetus was born to a GDM mother. There were 4 stillbirths in SCH group; the cause being RDS, birth asphyxia, meconium aspiration syndrome and the other one was unknown in whom the mother had GDM. Six and five neonates had congenital anomalies in euthyroid and SCH women respectively. The congenital anomalies present in euthyroid group were cloacal dystrophy, lateral ventricle dilatation, cleft lip and palate, anencephaly, fetal intraabdominal cystic lesion, and hypospadias. Similarly, the congenital anomalies present in SCH group were autosomal recessive polycystic kidney disease, bilateral hydroureteronephrosis, fetal right lung cystic lesion, choroid plexus cyst, and congenital diaphragmatic hernia. The proportion of still birth (aOR:12.01 (1.74-82.64)) was higher in SCH women with TPOAb as compared to euthyroid women as shown in Table 7.
DISCUSSION
A total of 178 pregnant women (87 euthyroid and 91 SCH) were recruited in the early pregnancy in this study. SCH women had higher BMI with more proportion of obesity compared to euthyroid subjects. High BMI during early pregnancy increases the risk of maternal thyroid dysfunction during pregnancy. The possible mechanism is due to the effect of adipokine like leptin on hypothalamus-pituitary-thyroid axis [26].
The neonatal intensive care unit (NICU) admission was higher (25/88 (28.4%) vs 10/82 (12.2%); p = 0.01) in women with SCH as compared to euthyroid women in this study. This can be explained due to increase proportion of obstetric complications among them such as GDM, Hypertensive disorders, oligohydramnios, IUGR and induced labor among women with SCH when compared to euthyroid women. The maternal BMI adjusted OR for NICU admission was 3.24 (1.41-7.43) in the SCH women. Otherwise, there was no difference in fetomaternal outcomes between the two groups. Similar result was found in a cross-sectional study by Sitoris [7]. The pregnancy outcomes were compared between 1281 euthyroid (TSH <2.51 mIU/L without thyroid autoimmunity) and 140 SCH (TSH 2.51-3.7 mIU/L) pregnant women. SCH mothers had higher risk of both NICU admission (aOR 19.36 (CI 1.18-316.97)) and LBW babies (21.38 (CI 1.29-353.39)). In a retrospective study by Arbib [6], 3231 euthyroid (TSH levels between 0.1mIU/L and 2.5 mIU/L) and 796 SCH (TSH levels between 2.5 mIU/L and 4 mIU/L) pregnant women were included. There was an increased risk (aOR 1.81, 95% CI 1.0-3.28) of only preterm delivery before 34 gestational weeks in SCH mothers compared to euthyroid subjects.
The need for both induced labor and NICU admission was more common among pregnant mothers with TPO Ab -ve SCH compared to euthyroid subjects in our study. Mothers with TSH 2.5 to 4.08 mIU/L and TPOAb-ve during early pregnancy was associated with higher risk of both miscarriages 1.58 (1.17-2.13) and maternal composite outcomes 1.27 (1.04-1.54) compared to euthyroid status (0.23 ≤ TSH ≤ 2.5 mIU/L) in a retrospective study by Zhang [8]. The occurrence of one or more of maternal outcomes was defined as the presence of maternal composite outcomes in their study. Except PIH (2.8 vs. 1.5%, OR = 2.99, 95% CI =1.24-7.23), no correlations were observed on the adverse pregnancy outcomes between the 971 euthyroid (0.27-2.5 mIU/L) and 433 SCH (2.5-4.0 mIU/L) TPO Ab -ve pregnant women, after adjustment for potential confounders in a study by Li [12]. Impaired endothelium-related vasodilation due to decreased production of nitric oxide is the possible mechanism of SCH induced PIH [27]. However, no differences in the prevalence of adverse pregnancy outcomes were observed between 172 SCH (2.5 < TSH ⩽4.0 mIU/l) and 2161 euthyroid (0.27 < TSH ⩽2.5 mIU/l) women among a TPO Ab negative population in a retrospective study by Zhu [13]. There were also no associations between TPOAb-negative women with TSH concentration between 2.5 and 4.0 mIU/L during their first trimesters and the incidences of adverse pregnancy outcomes in various studies [10-12]. However, the results were not controlled for other confounding factors in few of these studies.
The proportion of GDM, IUGR, and still birth was higher in SCH women with TPOAb as compared to euthyroid women in our study. Similarly, SCH with positive antithyroid autoantibodies markedly increased GDM risk (OR 3.22, 95% confidence interval 1.72-6.03, I2 =55%) in a meta-analysis by Jia [28]. Women with TSH levels >4.0 mIU/L have an increased odd of GDM regardless of thyroid autoimmunity status but at TSH levels <4.0 mIU/L, GDM is dependent on thyroid antibody status in a meta-analysis by Kent [22]. Presence of TPO Ab may lead to the progressive increase in TSH during pregnancy and thyroid hormone affects both insulin production from beta cells in islets and insulin sensitivity at peripheral tissue level [13,29]. This might be responsible for high prevalence of GDM among SCH pregnant women in presence of thyroid autoimmunity. TPO Ab has the ability to cross the placenta and affects fetal growth.30,31 Each 1 SD increase in maternal TSH concentration was associated with a 6 g lower birthweight (-10g to -2g; p=0·0030), with higher effect estimates in TPO Ab positive women than for women who were Ab negative as shown in a meta-analysis by Derakshan [21]. SCH is associated with IUGR (OR = 1.54; 95% CI, 1.06-2.25); however, TPOAb positivity does not affect the risk of IUGR as found in a meta-analysis by Tong [32]. This may be due to the sensitizing action of thyroid hormone on growth hormone and insulin like growth factor -1 affecting the fetal growth during intrauterine life irrespective of thyroid Ab status [33]. But, none of the fetomaternal outcomes was different between TPO Ab + ve SCH and euthyroid pregnant mothers in a study by Zhang [8]. The implications from our study is that SCH appears to have no influence on adverse pregnancy outcomes except a positive association with the NICU admission among them. There appears to be a higher proportion of overweight and obese women among the SCH women which emphasizes the need for counselling about diet and exercise. Advice regarding optimization of BMI prior to planning pregnancy prevents derangement of thyroid function in pregnancy and good feto-maternal outcomes.
Our study has few strength and limitations. This is the first study in South Indian population to evaluate pregnancy outcomes in women with SCH with and without TPOAb where there is lack of published data. As there was a difference in BMI among the study subjects it was adjusted in both euthyroid and SCH women to study the association in fetomaternal outcomes. In addition, the prospective nature of the study adds to the strengths. Since this study population was recruited from a tertiary hospital, the pregnancy outcomes may not reflect all women with SCH. The extent of derangement in thyroid function among women with SCH and association with NICU admission among babies born to them requires more prospective studies in the future with a large sample size. Moreover, in our study there is a small sample size in SCH women with TPO Ab and there is a lack of follow up of neurocognitive development of infants. We also could not estimate TPO ab status among euthyroid subjects.
CONCLUSION
There appears to be no difference in pregnancy outcomes between women with SCH and euthyroid women except higher NICU admission in SCH group. Our study is not powered enough to compare the effect of TPO Ab on fetomaternal outcomes. Future prospective studies with larger sample size are required to understand better about the pregnancy outcomes in SCH (TSH levels between 2.5-4mIU/L) with and without TPOAb.
FUNDING
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
COMPETING INTERESTS
The authors have no relevant financial or non-financial interests to disclose.
AUTHOR CONTRIBUTIONS
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Dr Priyanka R. The first draft of the manuscript was written by Dr Priyanka and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
ETHICAL PRINCIPLES
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the institution.
CONSENT TO PARTICIPATE
Informed consent was obtained from all individual participants included in the study.
CONSENT TO PUBLISH
No images of participants were used in the study.
- Yadav V, Dabar D, Goel AD, Bairwa M, Sood A, et al. (2021) Prevalence of Hypothyroidism in Pregnant Women in India: A Meta-Analysis of Observational Studies. J Thyroid Res 2021: 5515831.
- Dong AC, Stagnaro-Green A (2019) Differences in Diagnostic Criteria Mask the True Prevalence of Thyroid Disease in Pregnancy: A Systematic Review and Meta-Analysis. Thyroid 29(2): 278-289.
- Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, et al. (2011) Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 21(10): 1081-1125.
- Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, et al. (2017) Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 27(3): 315-389.
- Yang X, Meng Y, Zhang Y, Zhang C, Guo F, et al. (2019) Thyroid function reference ranges during pregnancy in a large Chinese population and comparison with current guidelines. Chin Med J (Engl) 132(5): 505-511.
- Arbib N, Hadar E, Sneh-Arbib O, Chen R, Wiznitzer A, et al. (2017) First trimester thyroid stimulating hormone as an independent risk factor for adverse pregnancy outcome. J Matern Fetal Neonatal Med 30(18): 2174-2178.
- Sitoris G, Veltri F, Kleynen P, Cogan A, Belhomme J, et al. (2020) The Impact of Thyroid Disorders on Clinical Pregnancy Outcomes in a Real-World Study Setting. Thyroid 30(1): 106-115.
- Zhang Y, Sun W, Zhu S, Huang Y, Huang Y, et al. (2020) The Impact of Thyroid Function and TPOAb in the First Trimester of Pregnancy Outcomes: A Retrospective Study in Peking. J Clin Endocrinol Metab 105(3): dgz167.
- Li MF, Ma L, Feng QM, Zhu Y, Yu TP, et al. (2020) Effects of Maternal Subclinical Hypothyroidism in Early Pregnancy Diagnosed by Different Criteria on Adverse Perinatal Outcomes in Chinese Women with Negative TPOAb. Front Endocrinol (Lausanne) 11: 580380.
- Rosario PW, Carvalho M, Calsolari MR (2016) TSH reference values in the first trimester of gestation and correlation between maternal TSH and obstetric and neonatal outcomes: A prospective Brazilian study. Arch Endocrinol Metab 60(4): 314-318.
- Veltri F, Kleynen P, Grabczan L, Salajan A, Rozenberg S, et al. (2018) Pregnancy outcomes are not altered by variation in thyroid function within the normal range in women free of thyroid disease. Eur J Endocrinol 178(2): 189-197.
- Li P, Lin S, Li L, Cui J, Zhou S, et al. (2018) Effect of mildly elevated thyroid-stimulating hormone during the first trimester on adverse pregnancy outcomes. BMC Endocr Disord 18(1): 64.
- Zhu P, Chu R, Pan S, Lai X, Ran J, et al. (2021) Impact of TPOAb-negative maternal subclinical hypothyroidism in early pregnancy on adverse pregnancy outcomes. Ther Adv Endocrinol Metab 12: 20420188211054690.
- Lee SY, Pearce EN (2022) Assessment and treatment of thyroid disorders in pregnancy and the postpartum period. Nat Rev Endocrinol 18(3): 158-171.
- Maraka S, Mwangi R, McCoy RG, Yao X, Sangaralingham LR, et al. (2017) Thyroid hormone treatment among pregnant women with subclinical hypothyroidism: US national assessment. BMJ 356: i6865.
- Ram U, Thirunavukkarasu M, Shyam K, Ghebremichael-Weldeselassie Y, Sukumar N, et al. (2023) Effects of treating subclinical hypothyroidism in pregnancy in India: Are we treating too many for little gain? A retrospective cohort studies. Int J Gynaecol Obstet 164(2): 677-683.
- Nazarpour S, Tehrani FR, Simbar M, Tohidi M, Majd HA, et al. (2017) Effects of levothyroxine treatment on pregnancy outcomes in pregnant women with autoimmune thyroid disease. Eur J Endocrinol 176(2): 253-265.
- Nazarpour S, Tehrani FR, Simbar M, Tohidi M, Minooee S, et al. (2018) Effects of Levothyroxine on Pregnant Women with Subclinical Hypothyroidism, Negative for Thyroid Peroxidase Antibodies. J Clin Endocrinol Metab 103(3): 926-935.
- Gietka-Czernel M, Glinicki P (2021) Subclinical hypothyroidism in pregnancy: controversies on diagnosis and treatment. Pol Arch Intern Med 131(3): 266-275.
- Han Y, Wang J, Wang X, Ouyang L, Li Y (2022) Relationship Between Subclinical Hypothyroidism in Pregnancy and Hypertensive Disorder of Pregnancy: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne) 13: 823710.
- Derakhshan A, Peeters RP, Taylor PN, Bliddal S, Carty DM, et al. (2020) Association of maternal thyroid function with birthweight: A systematic review and individual-participant data meta-analysis. Lancet Diabetes Endocrinol 8(6): 501-510.
- Kent NL, Young SL, Akison LK, Cuffe JSM (2021) Is the link between elevated TSH and gestational diabetes mellitus dependant on diagnostic criteria and thyroid antibody status: A systematic review and meta-analysis. Endocrine 74(1): 38-49.
- Liu H, Shan Z, Li C, Mao J, Xie X, et al. (2014) Maternal subclinical hypothyroidism, thyroid autoimmunity, and the risk of miscarriage: A prospective cohort study. Thyroid 24(11): 1642-1649.
- Misra A, Chowbey P, Makkar BM, Vikram NK, Wasir JS, et al. (2009) Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India 57: 163-170.
- Cunningham FG, Leveno JK, Bloom SL, Spong YC, Jodi S, et al. (2014) The thyroid disorders. In: Cunningham FG, Leveno JK, Bloom SL, Spong YC, Jodi S. Dashe, Hoffman LB, Brian editors. Williams Obstetrics. 25th New York: McGraw-Hill; pp: 637-653.
- Taddei S, Caraccio N, Virdis A, Dardano A, Versari D, et al. (2003) Impaired endothelium-dependent vasodilatation in subclinical hypothyroidism: beneficial effect of levothyroxine therapy. J Clin Endocrinol Metab 88(8): 3731-3737.
- Jia M, Wu Y, Lin B, Shi Y, Zhang Q, et al. (2019) Meta-analysis of the association between maternal subclinical hypothyroidism and gestational diabetes mellitus. Int J Gynaecol Obstet 144(3): 239-247.
- Mir F, Chiti H, Mazloomzadeh S (2022) Short-Term Adverse Pregnancy Outcomes in Women with Subclinical Hypothyroidism: A Comparative Approach of Iranian and American Guidelines. J Thyroid Res 2022: 9315250.
- Balucan FS, Morshed SA, Davies TF (2013) Thyroid autoantibodies in pregnancy: Their role, regulation and clinical relevance. J Thyroid Res 2013: 182472.
- Seror J, Amand G, Guibourdenche J, Ceccaldi PF, Luton D (2014) Anti-TPO antibodies diffusion through the placental barrier during pregnancy. PLoS One 9(1): e84647.
- Tong Z, Xiaowen Z, Baomin C, Aihua L, Yingying Z, et al. (2016) The Effect of Subclinical Maternal Thyroid Dysfunction and Autoimmunity on Intrauterine Growth Restriction: A Systematic Review and Meta-Analysis. Medicine (Baltimore) 95(19): e3677.
- Blazer S, Moreh-Waterman Y, Miller-Lotan R, Tamir A, Hochberg Z (2003) Maternal hypothyroidism may affect fetal growth and neonatal thyroid function. Obstet Gynecol 102(2): 232-241.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)
- Journal of Rheumatology Research (ISSN:2641-6999)
- Chemotherapy Research Journal (ISSN:2642-0236)
- Journal of Psychiatry and Psychology Research (ISSN:2640-6136)
- Journal of Ageing and Restorative Medicine (ISSN:2637-7403)
- Journal of Neurosurgery Imaging and Techniques (ISSN:2473-1943)
- Journal of Cancer Science and Treatment (ISSN:2641-7472)


