4
Views & Citations10
Likes & Shares
Thyrotoxicosis is a clinical condition characterized by an excessive increase in thyroid hormones with systemic repercussions. Surgical treatment of thyrotoxicosis is indicated when there is a poor response to medical management, suspicious cytology, iodine-induced thyrotoxicosis, and in patients who request it. However, prior to surgical intervention, it is essential to bring them to a euthyroid state in order to reduce perioperative cardiovascular risk and mortality [4,5]. That is why plasmapheresis has been used as a bridging therapy to stabilize the patient when conventional treatment fails and thus apply a subsequent definitive treatment.
METHODOLOGY
After taking the patient's complete clinical history and laboratory tests, a bibliographic search was carried out in different databases about plasmapheresis as a treatment in patients with thyrotoxicosis.
CLINICAL CASE
A 31-year-old man, resident of the department of Las Heras-Mendoza-Argentina, is presented.
He consulted the emergency department due to palpitations, weight loss of 34 kg in the last 3 months without hyporexia, distal tremor of the upper and lower limbs, proximal myopathy, generalized pruritus, WHO grade 2 goiter, sweating, heat intolerance and NYHA class II dyspnea with 2 months of evolution.
Given the risk of thyroid storm by Burch Wartofsky Score (BWS): of 30 points (heart rate 108 bpm, edema in lower limbs, diarrhea and mild agitation), and due to symptoms of thyrotoxicosis and increased liver enzymes (ALT x 1, 5), the patient was admitted to hospital begin to treatment and for control of the disease.
The patient was hypertensive (150/80 mmHg), with regular tachycardia 110 bpm, sweaty, afebrile, anxious, with generalized tremor, WHO grade 2 goiters, without regional lymphadenopathy, exophthalmos mild, and diarrhea.
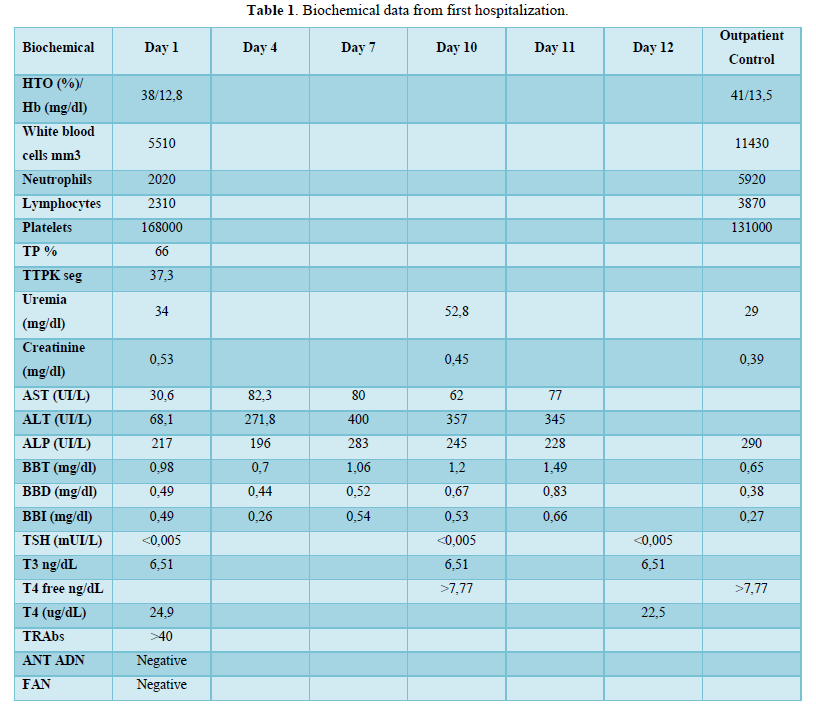
Biochemical tests showed a slight increase inALT, ALP and bilirubin (Table 1). Thyroid function tests were compatible with hyperthyroidism. Thyrotropin Receptor Antibodies (TRAbs) confirm Graves Basedow disease. (Table 1). Thyroid Ultrasound showed an overall enlarged gland with diffuse increase in vascularization. Right lobe 32cc, left lobe 18cc.
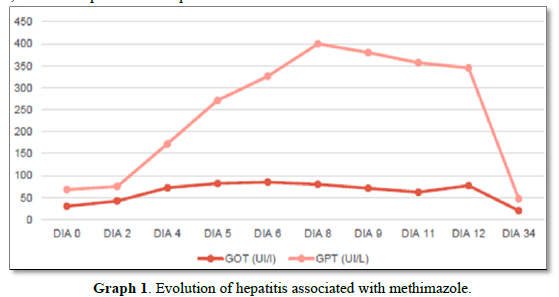
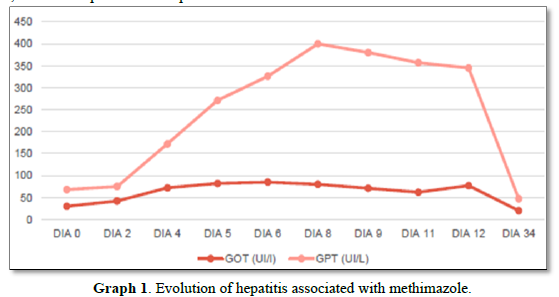
Despite the imminent risk of thyroid storm by BWS, low doses of methimazole (20 mg/day), was orally started as a precaution due to the initial alteration of the hepatogram that the patient presented. He also received atenolol 50 mg/8 h orally and intravenous dexamethasone 4 mg/8 h. The dose of methimazole is increased 24 h later to 40 mg/day. On the 4th day of treatment, he developed acute hepatitis due to methimazole, with coagulation alterations, and a normal liver ultrasound, so the drug was discontinued (Graph 1).
Oral lithium carbonate 300 mg/8 h was started, with prior informed consent, and continued with prednisone 20 mg/day, atenolol 50 mg/8 h, and losartan 25 mg/12 h. Due to clear improvement in the symptoms, discharge was decided on the 12th day, with a plan to achieve near normal level of thyroid hormones and then indicate total thyroidectomy.
Three days after discharge, he attended outpatient follow-up and due to lack of clinical and biochemical improvement, the dose of lithium carbonate was increased to 300 mg/6 h, the dose of prednisone was lowered to 10 mg/day, and atenolol was rotated to propranolol 40 mg/8 h. Lithemia was performed with a result of 0.6mmol/L (V.R. 0.6-1.20mmol/L).
Finally, he was re-admitted 10 days later to hospital, due to worsening symptoms that were interpreted as a lack of response to oral treatment; weight loss, proximal muscle weakness, functional class II dyspnea, sweating, asthenia, anxiety/nervousness, generalized tremors and diarrhea.
On physical examination he presented with tachycardia (115 bpm), hypertensive (150/80 mmHg), respiratory rate: 20 rpm, pulse oximetry 99%, temperature 37.3º. Homogeneous palpable goiter, without regional lymph nodes. Bilateral exophthalmos. Electrocardiogram: Sinus tachycardia. BWS 35 points (HR: 115 bpm, Tº: 37.3ºC, psychomotor agitation, and diarrhea).
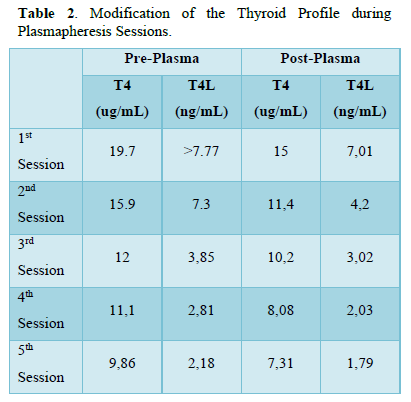
Given the intolerance to methimazole, the lack of response to medical treatment and the imminent risk of thyroid storm, it was decided to start plasmapheresis, as a bridge to surgical ablative treatment, using fresh frozen plasma as fluid replacement and completing 5 exchanges of 1 plasmalemma (4000mL). Free T4, calcium, potassium, clotting times and blood count were measured pre and post plasmapheresis.
After the 5th cycle of plasmapheresis (1 cycle daily) and with good tolerance, euthyroidism was achieved (Table 2) and thyroidectomy was performed 24 h after the last session, without complications. Thyroid biopsy report: DIFFUSE THYROID HYPERPLASIA LOB D: 62G:LI47GR.
DISCUSSION
In the case of our patient, acute toxic hepatitis induced by methimazole resulted in a contraindication to treatment with thionamides, which motivated the use of oral lithium carbonate. According to the current international consensus on drug-induced liver damage [10]. The standard definition of toxic liver damage meets the following biochemical criteria: ALT levels greater than or equal to 5 times the upper limit of normal (ULN), alkaline phosphatase levels (ALP) greater than or equal to 2 times the ULN or the combination of an increase in ALT greater than 3 times the ULN and total bilirubin greater than 2 times the ULN. Therefore, with the laboratory parameters obtained it was possible to confirm hepatotoxicity, considering the elevation of ALT more than 9 times the ULN (400 IU/l with a V.R <41 IU/L) and of ALP 2 times the ULN (283UI/L with V.R 40-126UI/L). Moreover, the biochemical classification of drug-induced liver injury, codified by the Council for International Organizations of Medical Sciences (CIOMS), classifies elevation of ALT>5 as hepatocellular damage.
It was decided not to use potassium iodide because there was no certainty of surgical date during the Covid 19 pandemic and we did not want to expose the patient to the possible phenomenon of iodine escape. Cholestyramine was not used because this drug was not available in our hospital.
The size of the gland and the presence of orbitopathy were the reasons why radioiodine treatment was not performed. The lack of response to lithium, the persistence of thyrotoxicosis symptoms, hepatotoxicity to methimazole and the imminent risk of thyroid storm were sufficient reasons to decide to perform plasmapheresis as a bridge therapy to thyroidectomy.
In reference to plasmapheresis or plasma replacement therapy (PRT), there are less than 100 reported cases in the literature and there are no randomized studies or clear guidelines on the indication criteria, and the best time to start this therapy and its duration are unknown. Although the indications for its use have not been standardized, agranulocytosis due to antithyroid drugs and presurgical preparation have been described in case reports [6,8].
TRP is a procedure used in thyrotoxicosis since 1970 [6]. It is performed using a cell separator. This equipment manages to separate the formed elements by centrifugation, removing the patient's plasma and replacing it with physiological solution, fresh frozen plasma or 5% human albumin, returning the cellular components.
The American Apheresis Association, in its latest guidelines, recommends this procedure in patients who are in thyroid storm or in those who are at high risk of developing it and do not respond to first-line pharmacological treatment, or this results in an adverse event [8].
Plasma exchange is a useful option in cases of severe hyperthyroidism unresponsive to antithyroid agents and in those with clinical manifestations of heart failure and in patients with serious adverse events during antithyroid therapy.
The principle of plasma exchange is based on removing large amounts of plasma in which free or protein-bound thyroid hormone is transported. In this case, the procedures were performed with fresh frozen plasma as a replacement fluid, since it contains thyroglobulin, a T4 transport protein, among other proteins, which reduce the amount of circulating thyroid hormone. However, this effect is temporary, so ablative treatment must be followed [9].
Some studies report that the decrease in thyroid hormones fluctuates by 20% after each session [7,8]. In our patient, the decrease in FT4, in the first session, was 41%, and it was reduced by 75% after 5 sessions with respect to the baseline value prior to the start of plasmapheresis [9] There are several complications that can occur during the TRP, but our patient did not present any of them.
Plasmapheresis, in the pre-surgery, reduces the risks of evolution to thyroid storm, a complication described in patients with uncontrolled thyrotoxicosis who undergo surgery or stressful situations [1]. Although admissions due to thyrotoxicosis or thyroid storm are not frequent, the mortality of these events reaches up to 30%, which justifies the aggressiveness of the treatment [7].
PRT can be considered a safe and effective alternative to prepare patients with thyrotoxicosis for surgery when pharmacological treatment fails or is contraindicated.
CONCLUSION
The metaverse affords individuals the chance to acquire a range of experiences in advanced digital technology. As the metaverse advances, its users expand quickly, prompting concerns over legal issues concerning illicit behaviors within the metaverse. However, the on-going legal dialogue on this topic remains inadequate.
On the other hand, South Korea is actively promoting regulatory reform in compliance with related legislation to promote the development of the metaverse industry and protect the rights and interests of users. This practice in South Korea is able to be one of the progressive legislation examples due to the limited regulations concerning the metaverse globally. As the legislation emphasizes self-regulation of the metaverse, which is considered a public sphere, it could lead to expedited settlement of disputes and growth of the metaverse industry. However, the important thing to consider is that as self-regulation is implemented without legal effect or binding force, unlike public regulation, there is the possibility of confusion in the application of the law as legal regulations. Besides, it needs to be discussed with additional studies about whether to deal with the legislation of the metaverse in terms of pre-regulation or post-regulation.
- Pereyra MC, Ramacciotti CF, Gecchelin RA, Braxs MC, Puccio RG, et al. (2020) Tratamiento de enfermedad de Graves con plasmaféresis. Rev Argent Endocrinol Metab 57: 22-25.
- Chair RSB, Burch HB, Cooper DS, Garber JR, Greenlee MC, et al. (2011) Hyperthyroidism and other causes of thyrotoxicosis: Management Guidelines of the American Thyroid Association and American Association of Clinica lEndocrinologists. Thyroid 21: 593-646.
- Galvis MVR, Ramón JGS, Mier HAB, Sánchez K, Mantilla W (2017) Uso exitoso de plasmaféresiscomo preparación para tiroidectomía en enfermedad de Graves no controlada y agranulocitosis inducida por metimazol. Revista Colombiana de Endocrinología y Metabolismo 4: 43-45
- Candoni A, De Marchi F, Vescini F, Mauro S, Rinaldi C, et al. (2017) Graves’ disease thyrotoxicosis and propylthiouracilrelatedagranulocytosissuccessfullytreatedwiththerapeutic plasma exchange and G-CSF followed by total thyroidectomy. Mediterr J Hematol Infect Dis 9(1): 1-5.
- Yamamoto J, Dostmohamed H, Schacter I, Ariano RE, Houston DS, et al. (2014) Preoperativetherapeuticapheresisforseveremedicallyrefractoryamiodarone-inducedthyrotoxicosis: A case report. J Clin Apher 29: 168-170.
- Ashkar FS, Katims RB, Smoak WM, Gilson AJ (1970) Thyroid Storm Treatment with Blood Exchange and Plasmapheresis. J Am Med Assoc 214: 1275-1279.
- Gómez C, Lina PC, Ramírez JO, Gómez P (2019) Plasmaféresis como terapia puente de la tiroidectomía en un paciente con tirotoxicosis. Revista Nefrología Argentina 17: 3-11.
- Schwartz J, Padmanabhan A, Aqui N (2016) Guidelines on the use of therapeutic apheresis in clinical practice-evidence- based-approach from the writing committee of the American Society for Apheresis: The seventh special issue. J Clin Apher 31: 149-162.
- Padmanabhan A, Connelly-Smith L, Aqui N, Balogun RA, Klingel R, et al. (2019) Guidelines on the Use of Therapeutic Apheresis in Clinical Practice-Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Eighth Special Issue. J Clin Apher 34: 171-354.
- López-P RdelP, Forero J-D, Sierra F (2014) Ictericia colestásica inducida por metimazol en una paciente con hipertiroidismo. Acta Gastroenterológica Latinoamericana 44(1): 52-58.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Advance Research on Endocrinology and Metabolism (ISSN: 2689-8209)
- BioMed Research Journal (ISSN:2578-8892)
- Journal of Pathology and Toxicology Research
- Journal of Allergy Research (ISSN:2642-326X)
- Archive of Obstetrics Gynecology and Reproductive Medicine (ISSN:2640-2297)
- International Journal of Diabetes (ISSN: 2644-3031)
- Journal of Ageing and Restorative Medicine (ISSN:2637-7403)