Review Article
A Review on Quorum-Sensing Inhibitors Derived from Plant Sources
5348
Views & Citations4348
Likes & Shares
Quorum Sensing (QS) is the process of chemical communication among bacteria to coordinate their activities necessary for their survival. It is a chemical signaling process through which bacteria recognizes information from other bacteria cells and regulates bacterial gene expression. QS inhibition has become an attractive project to control and combat the activity of multidrug resistant pathogens. Many natural occurring compounds derived from various plant sources can combat pathogens with unique chemical mechanism. Different plant extracts like alkaloids, flavonoids, phenolic compounds, quinones etc. have the unique property to control the pathogenic activity. In this review, anti-QS property of natural substances obtained from different plant sources are discussed briefly.
Keywords: Quorum Sensing, Inhibitors, Acyl homoserine lactone, Autoinducer, Plant Source
Abbreviations: QS: Quorum Sensing; QQ: Quorum Quenching; AHL: Acyl Homoserine Lactone; AIP: Auto Inducing Peptide; AI: Autoinducer
INTRODUCTION
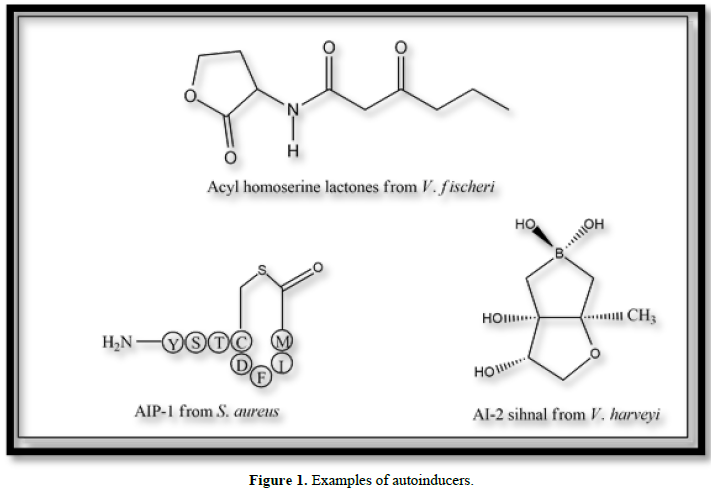
Bacteria communicate it cell to cell through a special signaling system called Quorum Sensing (QS). As an antimicrobial process, QS is a well-accepted and recognized target. The changes in population density of bacterial cell are detected by the production of autoinducer signal molecules and QS induces the specific gene expression. Anti QS is also known as Quorum Quenching (QQ) and it reduces the bacterial virulence and biofilm production. QQ is also advantageous since it does not produce drug resistance in bacteria owing to the fact that anti-QS does not impose any selection pressure. With the help of detection, diffusion, production and responses to chemical signaling molecules, communications among bacteria happens and are called autoinducers. With the increase in bacterial cell population AHL concentration reaches a specific level called threshold concentration. At this concentration level Autoinducers are detected. Thus, for the regulation of bacterial behavior like discharge and development of biofilm formation, virulence factors and also antibiotic production autoinducers are used in QS process. There is various type of signal molecules are produced by bacteria of which most common are i) Acyl homoserine lactone (AHL) - Lux R/I mechanism in gram-negative bacteria, ii) Auto inducing peptide (AIP) - in gram-positive bacteria and iii) Lux S /AI 2 in both gram-positive and gram-negative bacteria (Figure 1) [1].
QUORUM SENSING INHIBITION IN PLANTS
From the ancient ages, natural products especially extract from various plants were used in the treatment of various diseases. Plants do not have immune system like mammals and humans but they have developed unique anti-QS properties to control the pathogenic activity.
Recently, new medicines have been discovered for cure of various diseases by biologically active ingredients of natural products, mostly the once that are derived from plants [2]. In order to protect itself from the attack of pathogen, several studies proved that eukaryotes have developed efficiency to influence bacterial QS system [3]. It was first identified that the halogenated furanones produced by benthic marine microalga Delisea pulchra get in the way of the N-acylated homoserine lactones (AHL) regulatory system in a number of Gram-negative bacteria by competitively binding to LuxR type proteins. In the natural marine environment these QS. For example, lactone bond of AHL signaling compound was found to be hydrolyzed by N-acyl homoserine lactonase enzyme [6]. The paraoxonase (PON) enzyme which is present in epithelial cells of human airway also show anti QS property [7].
MECHANISMS
In order to prevent and treat contagious diseases, natural products play an essential role [8]. There are three different ways by which the plant compounds frequently aim the bacterial QS. These are by blocking the signaling molecules from being synthesized or by objectifying the LuxR signal receptor and by either discontinuing the signaling molecules [9].
A series of reaction are typically involved in AHL Biosynthesis that uses S-adenosyl methionine (SAM) as the amino contributor is generating the lactones ring moiety, and an acyl transporter protein (ACP) as the forerunner for the N-acyl side chain of the AHL molecules. Number of SAM analogues show anti QS actions and have been synthesized [10].
There can be non-competitive and competitive molecules which can interfere with the binding of AHL to cognate LuxR receptor to obstruct with signal reception. The molecules should be structurally identical to AHLs for competitive molecules to bond to AHL receptor. For competitive binding molecules binds to the site of the receptor. So, these molecules do not bind to AHL. For competitive binding, the molecules which are structurally similar to AHL are produced by the plants and hence they can bind to the AHL receptor [11]. Hence, QS inhibitors can also affect the integrity of biofilms and hence, it will make the bacteria more disposed to usual antibiotics [12]. To reduce the opportunity of the bacteria from becoming resistant this serves as an advantage [13].
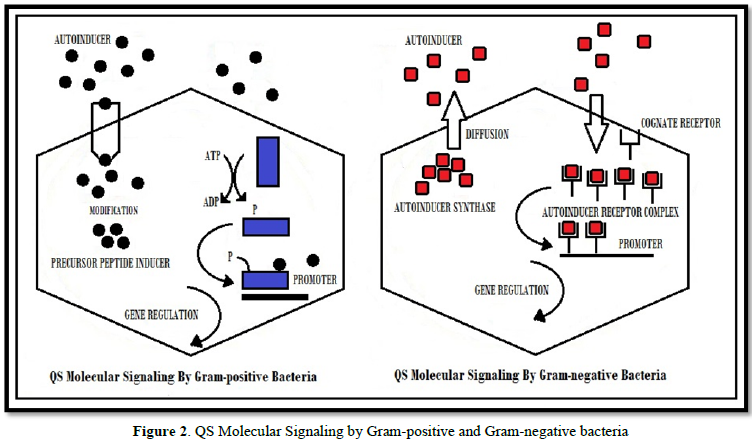
In gram-positive bacteria, auto inducing peptides are modified and transported out of the cell by ATP binding cassette exporter complex. When the threshold value of concentration of AIP reaches, the sensor kinase protein is activated and the response regulator protein is phospholyted. This protein is then bound to the target promoter which in turn leads to QS gene regulation.
In gram-negative bacteria, AI’s are synthesized and concentration of AIP is increased. When a particular concentration called threshold concentration, of AIP is reached, it diffuses out the cell walls of bacteria and a positive feedback loop is formed that causes more and more signaling molecules to be synthesized. The Ais approaches to the cognate receptor and bind to them to form auto inducer receptor complex. This complex moiety is then bind to the promoter. Finally, this results in QS gene regulation [14] (Figure 2).


The red marine algae which are also known as D. Pulchra is the most broadly studied natural compound. By interfering along with the AHL system, this Australian seaweed can manage bacterial colonization [15]. Halogenated furanones competitively bind to the LuxR type proteins in process to inhibit the QS regulated behaviors [16], as a result they enhance the rate of proteolytic degradation without killing the bacteria for their role in inhibiting biofilm formation [17]. Early researches show that for both AI-1 and AI-2 meditated QS system furanones are well-built inhibitors [16], which is partially relieved when the AHL absorption gets increased [15].
Beside red marine alga, grapefruit extracts also consist of a number of bioactive compounds such as limonoids, furocoumarins, pectin, carotenoids and coumarin which have antifungal and antibacterial actions [18]. Tough inhibitions against AI-1 and AI-2 activities are present in Furocoumarins. Moreover, the formation of biofilms in P. aeruginosa, S. typhimurium and E. coli is also hindered [19].
Other higher plants which are found to process anti-QS properties are vegetables. Anti QS actions against the luxl-gfp reported strain is present in chamomile, an array of peppers, water lily and even in carrot [11]. Also, obacunone has been proven to have a strong antagonistic activity against both AI-2 system and AHL, biofilm formation and EHEC virulence [20].
Moreover, it has been observed that the plants have the capability to degrade the signaling molecules produced by the bacteria and this will obstruct the bacteria virulence factors by disrupting their connection system [6]. There are certain fungi like Meliniomyces, Phialocephala and Ascomycete which are found in plant roots. These fungi can interfere with AHL and can control pathogenic activity [21].
Carvacrol is a compound which is found in many aromatic plants like oregano and it has anti-bacterial property. Carvacrol can reduce the production of violacein in C. violaceum ATCC 12472, Salmonella enterica subsp. Typhimurium DT104 and Staphylococcus aureus 0074 [22].
QUORUM SENSING INHIBITORS: FEW EXAMPLES
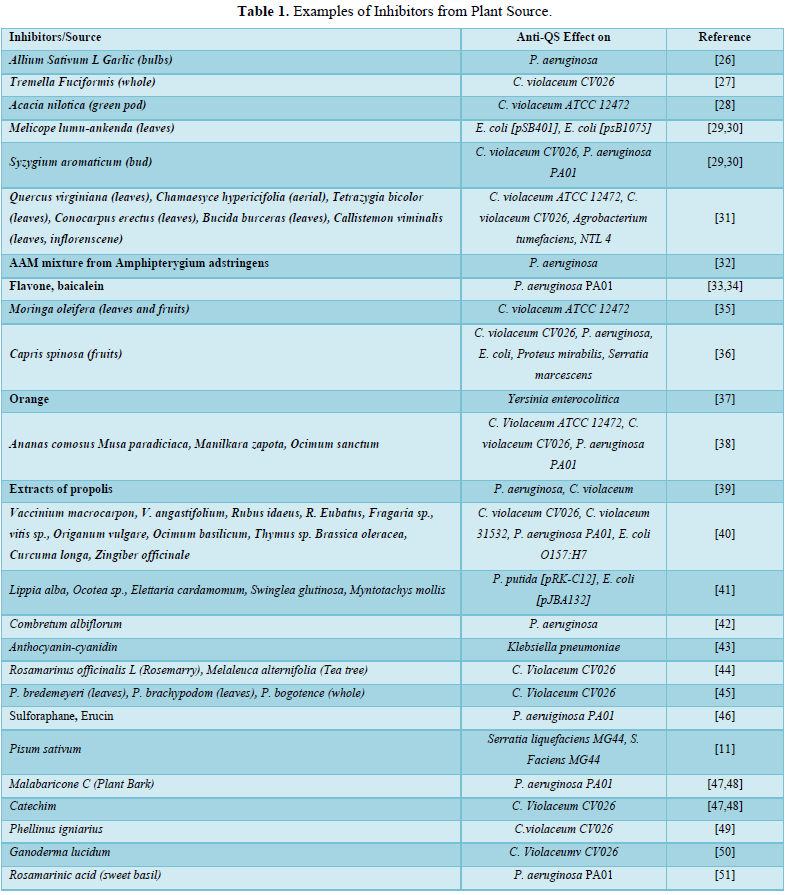
In human civilization, the uses of extracts from different plants where these compounds are mainly secondary metabolites and these are mainly phenols or oxygen-substituted phenols [23]. Oroidin, ursolic acid, cinnamaldehyde, salicylic acid, methyl eugenol, garlic extracts and also fruits which are constitute of plants, have the anti-biofilm properties towards various pathogens and bacteria [24]. Since these plants can be consumed by humans, the active compounds that are having QS inhibitory performances from the plants should be deemed as a secure and must not cause toxicity in the direction of human cells, but toxicity studies on these compounds are still required. Plants are capable of producing mimic molecules having anti-QS property. Besides that, there are certain plants or plant parts such as pea seed (Pisum sativum) which can ooze out liquids containing compounds having anti-QS property [11]. These observations lead to a better understanding about the process of inhibition of bacterial QS system. Researchers generally use biosensors for the screening of compounds which have anti-QS property. E. coli [pSB401], E. coli [pSB1075], C. violaceum CV026 etc. are used as biosensors. As these biosensors do not have the ability to produce AHL, it is supplied from outside and different QS traits like bioluminescence and violacein making are studied. The implication of the inhibition helped to calculate the anti - QS capability of compounds or extracts [25]. In the Table 1 below, some examples of inhibitors from plant sources are given -

CONCLUSION
In last few decades, several noble antibiotics are discovered to control the pathogenic activity. But the biggest challenge is the capability of bacteria to develop resistance against these drugs. Anti-QS will not likely cause resistance problems as it does not pose selection pressure and may be an important way to control antimicrobial activity. The examples cited here demonstrate the inhibition of virulence by inhibiting QS process. QS inhibition may be the future weapon to control the pathogenicity of various bacteria along with antibiotics. A new type of drug may be synthesized which would be cost effective and would have very few side effects.
CONFLICTS OF INTEREST
Authors declare that there are no potential conflicts of interest.
ACKNOWLEDGEMENT
The authors are grateful to the Departmental Special Assistance Scheme under the University Grants Commission, New Delhi and Head, all the members, Department of Chemistry, University of North Bengal, Darjeeling, India, for providing various facilities in connection with this review work.
- Ryan RP, Dow JM (2008) Diffusible signals and interspecies communication in bacteria. J Microbiol 154: 1845-1858.
- Hanson JR (2003) The Classes of Natural Product and Their Isolation; In Natural Products; The Secondary Metabolites; Royal Society of Chemistry; Cambridge, UK. pp: 1-34.
- Gozalez JE, Keshavan ND (2006) Messing with bacterial quorum sensing. Microbiol Mol Biol Rev 70: 859-875.
- Bauer WD, Teplitski M (2001) Can Plants manipulate bacterial quorum Sensing? Funct Plant Biol 28: 913-921.
- Kjelleberg S, Steinberg P, Givskov M, Gram L, Manefield M, et al. (1997) Do marine natural products interfere with prokaryotic AHL regulatory system? Aquat Microbial Ecol 13: 85-93.
- Dong YH, Wang LH, Xu JL, Zhang HB, Zhang XF, et al. (2001) Quenching quorum- sensing-department bacterial infection by an N-acyl homoserine lactonase. Nature 411: 813-817.
- Chun CK, Ozer EA, Welsh MJ, Zabner J, Greenberg EP (2004) Inactivation of a Pseudomonas aeruginosa quorum- sensing signals by human airway epithelia. Proc Natl Acad Sci USA 101: 3587-3590.
- Koehn FE, Carter GT (2005) The evolving role of natural products in drug discovery. Nat Rev Drug Discov 4: 206-220.
- Suga H, Smith KM (2003) Molecular mechanisms of bacterial quorum sensing as a new drug target. Curr Opin Chem Biol 7: 586-591.
- Parsek MR, Val DL, Hanzelka BL, Cronan JE Jr, Greenberg EP (1999) Acyl homoserine lactone quorum-sensing signal generation. Proc Natl Acad Sci USA 96: 4360-4365.
- Teplitski M, Robinson JB, Bauer WD (2000) Plants secrete substances that mimic bacterial N-acyl homoserine lactone signal activities and affect population density-dependent behaviors in associated bacteria. Mol Plant Microbe Interaction 13: 637-648.
- Dong YH, Gusti AR, Zhang Q, Xu JL, Zhang LH (2002) Identification of quorum-quenching N-acyl homoserine lactonases from Bacillus Appl Environ Microbiol 68: 1754-1759.
- Hentzer M, Givskov M (2003) Pharmacological inhibition of quorum sensing for the treatment of chronic bacterial infections. J Clin Invest 112: 1300-1307.
- Finch R, Pritchard D, Bycroft B, Williams P, Stewart G (1998) Quorum Sensing- A novel target for anti-infective therapy. J Antimicrob Chemother 42: 569-571
- Givskov M, Nys RD, Manefield M, Gram L, Maximilien R, et al. (1996) Eukaryotic interference with homoserine lactone-mediated prokaryotic signalling. J Bacteriol 178: 6618-6622.
- Manefield M, de Nys R, Kumar N, Read R, Givskov M, et al. (1999) Evidence that halogenated furanones from Delisea pulchra inhibit acylated homoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology 145: 283-291.
- Manefield M, Rasmussen TB, Henzter M, Andersen JB, Steinberg P, et al. (2002) Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology 148: 1119-1127.
- Heggers JP, Cottingham J, Gusman J, Reagor L, McCoy L, et al. (2002) The effectiveness of processed grapefruit-seed extract as an antibacterial agent: II. Mechanism of action and in vitro J Altern Complement Med 8: 333-340.
- Girennavar B, Cepeda ML, Soni KA, Vikram A, Jesudhasan P, et al. (2008) Grapefruit juice and its furocoumarins inhibits autoinducer signaling and biofilm formation in bacteria. J Food Microbiol 125: 204-208.
- Vikram A, Jesudhasan PR, Jayaprakasha GK, Pillai BS, Patil BS (2010) Grapefruit bioactive limonoids modulate coli O157:H7 TTSS and biofilm. Int J Food Microbiol 140: 109-116.
- Uroz S, Heinonsalo J (2008) Degradation of N-acyl homoserine lactone quorum sensing signal molecules by forest root-associated fungi. FEMS Microbiol Ecol 65: 271-278.
- Hani Z (2018) Anti-quorum sensing natural compounds. J Microsc Ultrastruct 6: 1-10.
- Choo JH, Rukayadi YH, Wang JK (2006) Inhibition of bacterial quorum sensing by vanilla extract. Lett Appl Microbial 42: 637-641.
- Abraham ISV, Palani A, Ramaswami BR, Shunmugiah KP, Arumugam VR (2011) Anti-quorum sensing and anti-biofilm potential of Capparis spinosa. Arch Med Res 42: 658-668.
- Koh C, Sam CK, Yin WF, Tan LY, Krishnan T, et al. (2013) Plant-Derived Natural Products as sources of Anti-Quorum Sensing Compounds. Sensors 13: 6217-6228.
- Bjarnsholt T, Jensen PO, Rasmussen TB, Christophersen L, Calum H, et al. (2005) Garlic blocks quorum sensing and promotes rapid clearing of pulmonary Pseudomonas aeruginosab Microbiology 151: 3873-3880.
- Zhu H, Sun SJ (2008) Inhibition of bacterial quorum sensing-regulated behaviors by Tremella fuciformis Curr. Microbiol 57: 418-422.
- Singh BN, Singh BR, Singh RL, Prakash D, Sarma BK, et al. (2009) Antioxidant and anti-quorum sensing activities of green pod of Acacia nilotica Food Chem Toxicol 47: 778-786.
- Tan LY, Yin WF, Chan KG (2012) Silencing quorum sensing through extracts of Melicope lunu-ankenda. Sensors 12: 4339-4351.
- Krishnan T, Yin WF, Chan KG (2012) Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa PA01 by Ayurveda spice clove (Syzygium aromaticum) bud extract. Sensors12: 4016-4030.
- Adonizio AL, Downum K, Bennett BC, Mathee K (2006) Anti-quorum sensing activity of medicinal plants in southern florida. J Ethnopharmacol 105: 427-435.
- Castillo-Juárez I, García-Contreras R, Velázquez-Guadarrama N, Soto-Hernández M, Martínez-Vázquez M (2013) Amphypterygium adstringens anacardic acid mixture inhibits quorum sensing-controlled virulence factors of Chromobacterium violaceum and Pseudomonas aeruginosa. Arch Med Res 44: 488-494.
- Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, et al. (1998) The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280: 295-298.
- Zeng Z, Qian L, Cao L, Tan H, Huang Y, et al. (2008) Virtual screening for novel quorum sensing inhibitors to eradicate biofilm formation of Pseudomonas aeruginosa. Appl Microbiol Biotechnol 79: 119-126.
- Singh BN, Singh BR, Singh RL, Prakash D, Dhakarey R, et al. (2009) Oxidative DNA damage protective activity, antioxidant and anti-quorum sensing potentials of Moringa oleifera. Food Chem Toxicol 47: 1109-1116.
- Abraham ISV, Palani A, Ramaswamy BR, Shunmugiah KP, Arumugam VR (2011) Anti-quorum sensing and antibiofilm potential of Capparis spinosa. Arch Med Res 42: 658-668.
- Truchado P, Giménez-Bastida JA, Larrosa M, Castro-Ibáñez I, Espín JC, et al. (2012) Inhibition of quorum sensing (QS) in Yersinia enterocolitica by an orange extract rich in glycosylated flavanones. J Agric Food Chem 60: 8885-8894.
- Musthafa KS, Ravi AV, Annapoorani A, Packiavathy IS, Pandian SK (2010) Evaluation of anti-quorum-sensing activity of edible plants and fruits through inhibition of the N-acyl-homoserine lactone system in Chromobacterium violaceum and Pseudomonas aeruginosa. Chemotherapy 56: 333-339.
- Lamberte LE, Cabrera EC, Rivera WL (2011) Activity of the ethanolic extract of propolis (EEP) as a potential inhibitor of quorum sensing-mediated pigment production in Chromobacterium violaceum and virulence factor production in Pseudomonas aeruginosa. Agric Sci 94: 14-22.
- Vattem DA, Mihalik K, Crixell SH, McLean RJ (2007) Dietary phytochemicals as quorum sensing inhibitors. Fitoterapia 78: 302-310.
- Jaramillo-Colorado B, Olivero-Verbel J, Stashenko EE, Wagner-Dobler I, Kunze B (2012) Anti-quorum sensing activity of essential oils from colombian plants. Nat Prod Res 26: 1075-1086.
- Vandeputte OM, Kiendrebeogo M, Rasamiravaka T, Stévigny C, Duez P, et al. (2011) The flavanone naringenin reduces the production of quorum sensing-controlled virulence factors in Pseudomonas aeruginosa Microbiology 157: 2120-2132.
- Gopu V, Shetty PH (2016) Cyanidin inhibits quorum signaling pathway of a food borne opportunistic pathogen. J Food Sci Technol 53: 968-976.
- Alvarez MV, Moreira MR, Ponce A (2012) Antiquorum sensing and antimicrobial activity of natural agents with potential use in food. J Food Safety 32: 379-387.
- Olivero JTV, Pajaro NPC, Stashenko E (2011) Antiquorum sensing activity of essential oils isolated from different species of the genus Piper. Vitae 18: 77-82.
- Ganin H, Rayo J, Amara N, Levy N, Krief P, Meijler MM (2013) Sulforaphane and erucin, natural isothiocyanates from broccoli, inhibit bacterial quorum sensing. Med Chem Commun 4: 175-179.
- Chong YM, Yin WF, Ho CY, Mustafa MR, Hadi AH, et al. (2011) Malabaricone c from Myristica cinnamomea exhibits anti-quorum sensing activity. J Nat Prod 74: 2261-2264.
- Vandeputte OM, Kiendrebeogo M, Rajaonson S, Diallo B, Mol A, et al. (2010) Identification of catechin as one of the flavonoids from Combretum albiflorum bark extract that reduces the production of quorum-sensing-controlled virulence factors in Pseudomonas aeruginosa Appl Environ Microbiol 76: 243-253.
- Zhu H, Liu W, Wang S, Tian B, Zhang S (2012) Evaluation of anti-quorum-sensing activity of fermentation metabolites from different strains of a medicinal mushroom, Phellinus igniarius. Chemotherapy 58: 195-199.
- Zhu H, Liu W, Tian B, Liu H, Ning S (2011) Inhibition of quorum sensing in the opportunistic pathogenic bacterium Chromobacterium violaceum by an extract from fruiting bodies of lingzhi or reishi medicinal mushroom, Ganoderma lucidum (w. Curt.: Fr.) p. Karst. (Higher basidiomycetes). Int J Med Mushrooms 13: 559-564.
- Corral-Lugo A, Daddaoua A, Ortega A, Espinosa-Urgel M, Krell T (2016) Rosmarinic acid is a homoserine lactone mimic produced by plants that activates a bacterial quorum-sensing regulator. Sci Signal 9: ra1.




