2668
Views & Citations1668
Likes & Shares
Brain drug delivery strategies have not been widely used because they are risky, costly and unsuitable for less localized brain diseases [6]. The strategy of blood-to-brain delivery that involves improving BBB permeability of drugs or drug-carrier conjugates under normal conditions can minimize the above-mentioned side effects [7-8]. Nano materials have been widely investigated for brain drug delivery; in this review paper, we discuss various forms of Nano materials for blood-to-brain drug delivery through the intact BBB; the mechanisms of Nano materials-mediated drug transport across the BBB; and the future directions of this innovative area of research.
The passage of molecules through BBB depends on their structure, surface properties and chemical composition, allowing only low molecular weight (< 400-500 Da) and small lipophilic molecules to enter the brain with several folds of greater competence than large molecules [9]. The structure and function of BBB can be changed in neurodegenerative disease; the barrier function of BBB is still generally stable in the treatment neurodegenerative diseases [10,11].
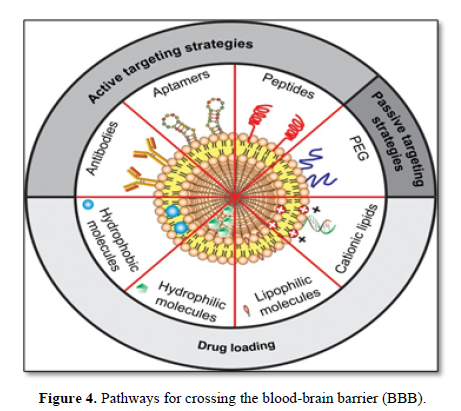
Prime significance to investigate different vehicles which can enhance the BBB transportability of therapeutic drugs to target area the cationic vehicle crosses the BBB via absorption mediated transcytosis. Some examples of commonly used Nano carriers are liposomes, nanoparticle, Nano micelles and exosomes. Drug delivery using Nano carriers may increase the bioavailability and stability of drugs and decrease the peripheral toxicity [12].
Nanotechnology represents the capacity to understand, manipulate, and control the matter at the level of individual atoms and molecules [13]. Therefore, the indication of nanotechnology for the development of non-invasive drug delivery strategies could lead to the design of novel and improved formulations to enhance the delivery of therapeutic agents across the blood-brain barrier Numerous research studies have focused on the exploration of nanotechnology-based drug delivery systems, including nanoparticle, liposomes, dendrimers, carbon nanotubes, and micelles, which have the potential to deliver the desired quantity of the drug to the brain [14-16].
Barriers to Drug Delivery for the CNS-Disease
The CNS permeability of a drug is determined by the drug’s ability to cross the blood-brain barrier (BBB). The brain is one of the most complex and important organs of living organisms. Therefore, it is necessary to protect it against the contamination with environmental and foreign substances which could lead to changes in the inner and outer
Concentrations of neuronal cells and subsequently to impairments in nerve conduction and dysfunctions in the body control processes [17]. The brain is a uniquely protected organ residing within the bony confines of the skull, thus making systemic delivery of drugs difficult. Barrier layers are formed at three interfaces: blood vessels of the brain (blood brain barrier), the choroids plexus (Blood cerebrospinal fluid barrier) and the arachnoids layer of the meninges (blood–arachnoids layer) [18-19] (Figure 1).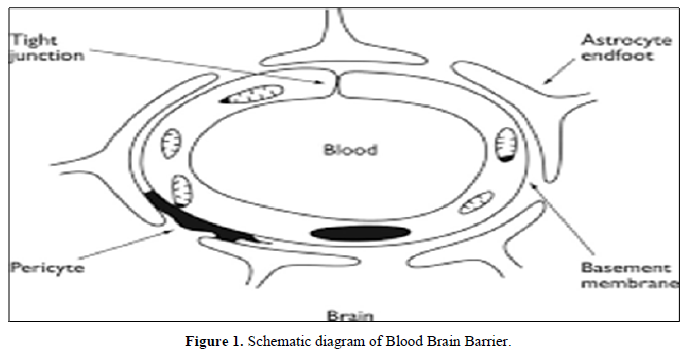
The blood-brain barrier is the structure responsible for the protection of the brain, acting as a local gateway against the circulating toxins and cells through a selective permeability system. Brain capillary endothelial cells (BMEC), together with astrocytes, pericytes, neurons, and the basal lamina, constitute the neurovascular unit, the functional units of the BBB, which maintain the homeostasis of the brain microenvironment [20]. Nano carriers are small-size particles used for the controlled delivery of pharmaceutical agents that are encapsulated within the mentioned Nano carriers, and adsorbed or conjugated onto their surface [21].
Nanoparticle for Drug Delivery to the Brain
Nanoparticle for drug delivery to the brain is a method for transporting drug molecules across the Blood Brain Barrier (BBB) using nanoparticle. These drugs cross the BBB and deliver pharmaceuticals to the brain for therapeutic treatment of neurological disorders. These disorders include Parkinson’s disease, Alzheimer’s disease, schizophrenia, depression, and brain tumors. Part of the difficulty in finding cures for these central nervous system (CNS) disorders is that there is yet no truly efficient delivery method for drugs to cross the BBB. Antibiotics, Anti-neoplastic agents, and a variety of CNS-active drugs, especially neuropeptides, are a few examples of molecules that cannot pass the BBB alone [22] with the aid of nanoparticle delivery system; however, studies have shown that some drugs can now cross the BBB, and even exhibit low toxicity and decrease adverse effects throughout the body. Toxicity is an important concept for pharmacology because high toxicity levels in the body could be detrimental to the patient by affecting other organs and disrupting their function [23]. Further, the BBB is not only the barrier for drug delivery to the brain. Other biological factors affect how drugs are transported to the body and how they target specific locations for action. Some of these pathophysiological factors include blood flow alterations, edema and increased intracranial pressure, metabolic perturbations, and altered gene expression and protein synthesis [24] though there exist many obstacles that make developing a robust delivery system difficult, and nanoparticle provide a promising mechanism for drug transport to the CNS.
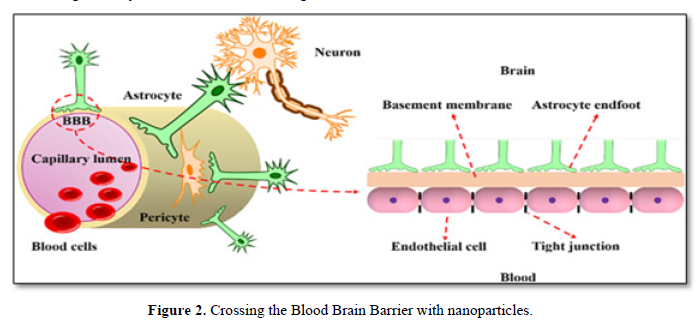
Nano carriers are small-size particles used for the controlled delivery of pharmaceutical agents that are encapsulated within the mentioned Nano carriers, and adsorbed or conjugated onto their surface [25]. Various materials may be used as Nano carriers; these includes [26] liposomes, micelles, polymeric and lipid-based nanoparticle, dendrimers, and carbon nanotubes. The average size of cells in the human body is 10-20μm; thus, adsorption or uptake of the Nano-sized drug–carrier conjugates by cells is possible, providing an opportunity to deliver drugs into cells. With the possibility of being surface functionalized with targeting ligands, Nano carriers offer the capability of transporting drugs across the BBB dendrimers and carbon nanotubes (Figure 2).
Liposomes: Classic Dosage Form to Penetrate BBB
Liposomes are Nano-sized vesicles with an aqueous inner core enclosed by unilamellar or multilamellar phospholipids bilayers. Liposomes have been widely investigated for systemic delivery of therapeutics [27]. Liposomes (LPs) are delivery systems with the capacity to encapsulate a large number of drugs and imaging agents. The structure of LPs allows for the delivery of a cargo loaded in the aqueous compartment or embedded in the lipid bilayers. They were the first generation of novel carriers for drug delivery [28]. Liposomes with structure similar to cell membrane are biodegradable colloids and can be employed to carry a wide range of hydrophobic and hydrophilic pharmaceuticals, such as small molecules peptide, proteins and RNAs, without changing their function and protecting them against degradation and potential immune responses (Figure 3). The glucose moiety of the ligands provided special affinity of the liposome with BBB endothelial cells, leading to an improvement of the transport rate. Doxorubicin liposomes conjugated with both foliate and transferrin also showed effectiveness in penetrating the BBB and targeting brain tumors [29]. The brain delivery of liposomes is the low stability, as well as the difficulty of binding ligands to the surface as a result of the small number of available surface groups and steric hindrance.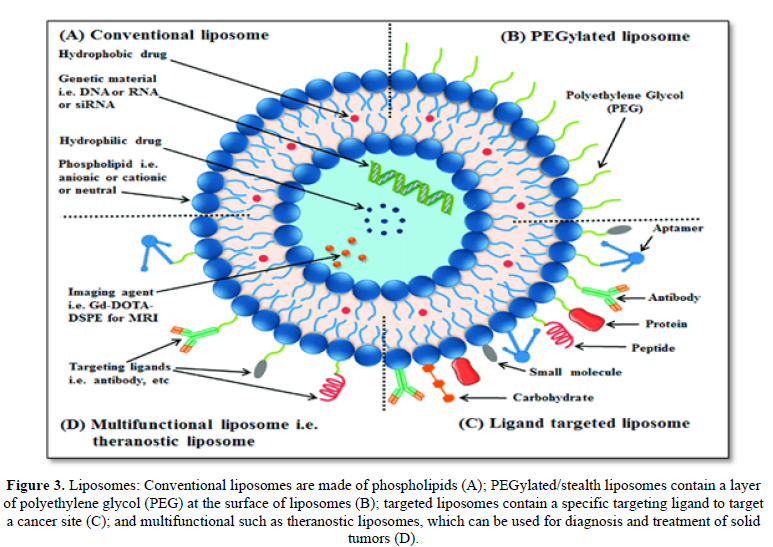
There are several limitations of liposomes, including fast systemic elimination, quick metabolic degradation of the phospholipids, and stability issues after extended storage, inability to provide sustained release of drugs and moderately efficient for the entrapment of lipophilic compound [30]. Initial studies employing very large liposomes were quite unsuccessful in transporting drugs across the BBB. When fluorescein or trepan blue were encapsulated in liposomes and injected intravenously, they stained only the luminal side of the vasculature and not the luminal side or brain parenchyma, indicating failure of liposomes to cross the BBB [31]. After recognizing that the relatively large size of liposomes i.e., 0.2- 1.0 µm was responsible for rapid ingestion by the cells in the reticule-endothelial system (RES), particularly in liver and spleen, liposomes were subsequently designed that were small unilamellar vesicles (SUVs), which ranged from 0.025 to 0.1 µm in diameter [32]. The use of SUV liposomes was found to greatly retard the rate of clearance from blood as compared with large vesicles. Subsequently, most of the research on liposomes for the BBB delivery has focused on the use of these SUVs.
Several studies have demonstrated utility of liposomes in the BBB penetration in the treatment of cerebral ischemia. Among these studies were included encapsulation of ATP, Calpain inhibitor, antioxidants (ascorbic acid and α-tocopherol), CDP-choline, citicholine and superoxide dismutase in liposomes and their effectiveness to control ischemia induced neuronal damage when compared with free drug administration (Figure 3).
Transferrin Modified Liposomes
Transferrin receptors as one of receptors are of special grip for delivery therapeutic agents across BBB in order to enhance the targeting efficiency. The receptor is a trans-membrane glycoprotein with two subunits of 90kDa that are enclosed by a disulfide bridge, and each of these subunits can bind to one molecule of transferrin (Figure 4). However, as a target drug delivery system there are some problems about transferrin must be focused.
Transferrin is also expressed on hepatocytes, monocytes, erythrocytes, intestinal cells of choroid plexus and neurons besides BBB. Thus, Transferrin targeted liposomes also have high distributions in liver and kidneys [33].
Transferrin receptor regulates the uptake of iron into the brain parenchyma. It has been observed that transferrin functionalized fluorescein-loaded magnetic NPs (FMNs) were found located in the dendrites, synapse of neurons, cytoplasm and axons, indicating that they can effectively cross the intact BBB through transferrin.
Micelles
Polymeric micelles have emerged as candidates for brain targeting delivery. Their use as targeted delivery drugs and as diagnostic-imaging agents has gained much interest, since they have a number of physical and bio-chemical advantages over other types of Nano carriers [34].
Their particle size is usually within a range of 10 to 100 nm, and most of these amphiphilic polymers are biodegradable and biocompatible. Micelles serve as are excellent pharmaceutical carriers due to their ability to solubilize hydrophobic drugs, prevent drug degradation, increase their water solubility, extend their circulation time, biocompatibility, and have lower adverse side effects [35]. Amphiphilic surfactant molecules that spontaneously aggregate in water into a spherical vesicle consist of a shell and a core. Poloxamers, micelles made up of Pluronic block copolymers, they are the most studied micelles Nano carriers [36]. The early drug release from the micelle Nano system before it reaches its specific targets can be prevented by micelle fictionalizations, which increase their stability and improves their time in circulation [37].
Nanoparticle
Nano particles are colloidal systems with compact / structure where the therapeutic agent is either entrapped within the colloid matrix or coated on the particle surface by conjugation or adsorption. The reason why nanoparticles are being widely used in treating neurodegenerative diseases is that they show following distinct characteristics:
- Nano-particles possess relatively high drug loading and small size and deliver the active ingredient to the specific site at a controlled and sustained rate during the transportation.
- Nano-particles, especially inorganic nanoparticle, show excellent imaging performance.
These Nano carriers can be classified as polymeric or lipid-based NPs. Nanoparticle are defined as solid colloidal particles made of polymeric materials ranging in size from 1-1000 nm [38-40]. They are used as drug carrier system in which the active compound is dissolved, entrapped, encapsulated and /or to which the active compound is adsorbed or attached. Examples of synthetic polymers used to prepare nanoparticles are poly (methyl methacrylate), poly (alkylcynoacrylate), acrylic copolymers, poly (D, L-lactide-co-glycoside), and poly (lactide) [41]. Nanoparticles have also been produced from natural proteins (albumin and gelatin) and polysaccharides (dextran, starch, and chitosan). Physicochemical studies have shown that coating of colloidal particles with block copolymers such as poloxamers and polyamines induced a steric repulsion effect, minimizing the adhesion of particles to the surface of macrophages, which in turn resulted in the decrease of phagocytes uptake and in significantly higher levels in the blood and non-RES organs including brain, intestine, and kidneys among others [42,43].
Lipid-based NPs, such as solid lipid nanoparticle (SLNs), are an important class of colloidal systems in the area of modified drug delivery technology. SLNs are made from one solid lipid, while NLCs are from a mixture of spatially different lipids or from a blend of a solid lipid with a liquid lipid (oil) [44]. Even if SLNs have low loading capacity for hydrophilic drugs, they are considered as very attractive Nano vectors for delivering drugs to the brain, because of their lower toxicity and high biodegradability. They are generally made up by a hydrophobic core containing the drug, which is either dissolved or dispersed [45]. SLN include widely used food lipids and waxes and commonly used emulsifiers include different kinds of poloxamers, Polysorbate, lecithin, and bile salts.
Polymeric NPs pass through the BBB by endocytosis followed by transcytosis through the BMEC lining the blood capillaries of the brain 4 Drugs delivered to the CNS by polymeric NPs are P-gp substrates, which are actively exported from the CNS. The use of polymeric nanoparticles increases drug delivery to the brain, with reduced oxidative stress, inflammation and plaque load through the improved delivery of curcumin for treating Alzheimer’s disease [46,47] Additionally, the in vivo experiment regarding the co-delivery of Cisplatin and Boliden, an antioxidant agent, using the poly (lactide-co-glycolic) Nano carriers resulted in an effective target-specific delivery for therapeutic use in brain cancer therapy [48]. In brief, it is possible that nanoparticle transport drugs across the BBB by any one, or combination of mechanisms.
Dendrimers
Dendrimers are versatile and highly branched structures consisting of repeating monomer units that are attached to and around a central core. Dendrimers possess exceptional structural properties such as small size, minimal polydispersity, narrow molecular weight distribution, well-defined globular shape, and a relative ease incorporation of targeting ligands, which make them attractive candidates for drug delivery [49]. The surface groups of dendrimers can be conjugated with ligands for transport across the BBB, as well as for targeting specific cells such as tumor cells. Thus, dendrimers are promising tailor able delivery systems for improved delivery of drugs to the brain. The drug-dendrimers conjugate showed a fast drug release profile at weak acidic condition and a stable state at physiological environment, as well as a good BBB transportation ability with the transporting ratio of 6.06% in 3h. Before translation of Dendrimers into brain drug delivery use, it is necessary to address their biocompatibility issue. For example, PAMAM Dendrimers have been shown to be hemolytic and Cytotoxic [50]. Some research results also showed that biotinylated PAMAM Dendrimers may prove to be more toxic compared to PAMAM Dendrimers alone [51]. One of the limitations of dendrimers is the variability of release mechanisms; drugs tend to be released before the Dendrimers can reach their target sites. Furthermore, their long-term safety profiles are relatively less established than other polymers [52].
Exosomes New Emerging and Promising Nanocarriers
Exosomes, as one of natural endogenous nanocarriers, vary from 30nm to 150nm in size and have a typical lipid bilayers structure, which are reputed as “drifting bottle” in living body. It is secreted by a variety of cells: B cells, and T cells, Macrophages, dendrite cells [53].
Exosomes distinguish themselves from other vehicles mainly in two features. The one is immune privilege: as natural carrier systems with endogenous cellular tropism, exosomes can avoid the endosomal pathway and liposomal degradation, diminish clearance by the mononuclear phagocyte system, and increase drug transport to target tissue, just functions as “invisibility cloak”. Intercellular communication over vast distances makes it easier to transport proteins and nucleic acids, which are unstable medicinal agents [54,55]. Exosomes, may represent a substantial advancement in the field of macromolecular drug delivery and may be a crucial step in the therapeutic use of SiRNA, while the use of exosomes as SiRNA vectors is still in its infancy. The cause-and-effect relationship between exosomes and pathogenesis of AD and PD is not clear enough related research is still at early stage. There are several problems waiting to be handled before they enter into clinical practice:
- Exosomes are so complex in molecular constituents that safety issues and potential risk must be highlighted and evaluated comprehensively.
- As brain target vehicle, it can’t be overemphasized to improve the target ability of exosomes to enhance the drug concentration in brain area and avoid adverse effect. Proof of concept has been gained for exosomes-based brain drug delivery systems; several issues should be addressed before clinical evaluation such as the choice of exosomes donor cells, drug loading procedures, as well as the targeting peptides.
Quantum dots
Quantum dots are a class of colloidal semiconductor nano crystals composed of a metalloid crystalline core (such as cadmium selenium) and an intermediate uncreative metallic shell (such as zinc sulfide) that shields the core [56]. The outer coating of quantum dots can be chemically functionalized with bioactive molecules that promote aqueous solubility and desired bioactivity, enable targeting of specific molecules, as well as carrying therapeutic molecules [57].
It is widely known that the transferrin receptor is a kind of specific BBB transporter that allows selected bimolecular to move across the BBB; lysine-coated CdSe/CdS/ZnS quantum dots have been synthesized followed by conjugation with transferrin while, Captopril-conjugated CdSe/ZnS-core/shell-typed quantum dots (QDs-cap) have been synthesized by the hot soap method with tri-n-octal phosphate oxide (TOPO) followed by the replacement of TOPO with Captopril by a thiol exchange reaction.
TAT, a cell membrane translocation peptide, has been successfully used to internalize nanoparticle. Research has shown that TAT-conjugated CdS/Mn/ZnS quantum dots can label the brain tissue within a few minutes after being intra-arterially delivered to a rat brain without manipulating the BBB; that type of TAT-conjugated quantum dots migrated beyond the endothelial cell line and reached the brain parenchyma. Because the same quantum dots without TAT did not label the brain tissue, TAT peptide was necessary for the quantum dots to overcome the BBB [58,59].
Nano emulsions
Nano emulsions are heterogeneous dispersions of oil-in-water (O/W) or water-in-oil (W/O) formulations stabilized with surface-active agents, where diameter of the inner phase is reduced to nanometer length scale. For biocompatibility purpose, Nano emulsions are usually made from edible oils, such as flaxseed oil, pine-nut oil, hemp oil, fish oil as well as safflower oil and wheat-germ oil, biocompatible surfactants such as egg phosphate dichloride which is one of the components of cell membrane lipids, deoxycholic acid, Stearylamine, dioleoyl tri-methylammonium propane (DOTAP) and water. The versatility of Nano emulsions is based on the different types of oils and surface modifiers that can be used [60].
Emulsions which have droplet sizes between 5-200 nm are named as Nano emulsions, ultrafine emulsions, submicron emulsions, translucent emulsions and mini-emulsions [61]. Nano emulsions are developed systems for the delivery of biologically active agents for controlled release and drug delivery. They are promising systems for the fields of cosmetics, diagnostics, drug therapy and biotechnology [62]. Moreover, they possess great potential as a novel delivery system in food industry for fatty acids, polyphenols, natural colors, and flavors especially for producing functional foods [63]. Lipophilic active compounds have poor water solubility and thus introducing them into food and beverages is a big challenge for food industry. Using Nano emulsions as a carrier system solve the solubility problem and also increase bioavailability of lipophilic active compounds such as vitamins and carotenoids [64].
Carbon Nanotubes
Carbon-based materials such as fullerenes and nanotubes may be advantageous in biotechnological applications for the variety of properties and shapes that they offer [65]. Carbon nanotubes (CNTs) are cylindrical structures formed by graphite sheets with a diameter in the nanometer range; thus, their transport across the BBB is facilitated. It is known that this process plays a key role in the Nano carrier’s toxicity. Functionalization requires a profound knowledge of the target organ and its transport mechanism [66].
Carbon nanotubes can be single-walled or multi-walled, with open ends or closed with fullerene caps [67]. The permeation of amino-functionalized multi-walled carbon nanotubes through the blood-brain barrier has been studied in vitro, by using a co-culture model comprising primary porcine brain endothelial cells, primary rat astrocytes, and in vivo, through the systemic administration in mice. The results of the study could pave the way for carbon nanotubes application in the delivery of drugs and biologics to the brain, causing no toxic effects on the cells [68].
Nano gels
Nano gels are novel formulation of nanoparticles and offer the prospect of drug transport across the intact BBB. It has been reported that Nano gels with surface charge exhibited better internalization property on cell membrane than neutral ones; Gil and Lowe synthesized polysaccharide-based Nano gels containing poly (B-amino ester) and B-cyclodextrin for transporting doxorubicin and insulin across the BBB, such cationic Nano gels enhanced the permeability of insulin across the in vitro BBB model by 20% [69]. It is well established from the literature that lipophilic moieties easily penetrate the blood brain barrier in compared to hydrophilic ones; so surface functionalization towards lipophilicity of Nano gels has been introduced as an accelerated encapsulated drug transport across the blood brain barrier. Azadia [70] prepared nano gels loaded with methotrexate (MTX) via an ionic gelation process using chitosan and sodium tripolyphosphate (TPP) as raw materials, the surfaces of the MTX-loaded Nano gels were modified with polysorbate 80 to improve brain drug delivery.
Limitations of Brain Targeted Nanoparticles
Even if nano-carriers are associated with various advantages like capability of transporting drugs across BBB or increased retention time in the circulatory system, their applications in a clinical scenario are restricted due to certain limitations. Foremost concern associated is the toxic effects exerted by the overexposure of nanomaterial, such as polymers. Owing to the major compositional percentage of Nano-drugs, polymers may get accumulated in the CNS due to repeated administration of Nano-carriers. It can cause both toxicity and immunogenicity. Therefore, rigorous regimes of experimental protocols are required to address as well curtail these problems before clinical implications. Primarily, it is indispensable to study the long-term toxicity profile of NPs in the brain, as it may limit the application of Nano-drug in clinics. Secondly, during the scaling up of the Nano-drug formulation process, from laboratory to industrial production, it is essential to maintain the encapsulation efficiency rate. The clinical efficiency of Nano-drugs may vary depending on their formulation and physicochemical properties while conjugating or encapsulation drugs. Therefore, the formulation process optimization for large scale production is crucial for maintaining the encapsulation efficiency pace during physiological conditions. Additionally, the high cost of scaling up the Nano-drug synthesis process and the use of organic solvents during the synthesis of Nano-drugs also confines the process. Therefore, there is a need to find alternatives for producing cost effective and ecofriendly Nano materials. As another limitation, the use of pH dependent fluorescent tags (i.e., FITC) for the detection of exocytose nanoparticles may interfere with the interpretation of results. The use of natively fluorescent (quantum dots, Nano diamonds), luminescent (gold) and paramagnetic (ferrous oxide) can offer distinct advantages to overcome this drawback.
CONCLUSION
Nano-carriers have been widely studied as drug delivery vehicles for transporting drugs to various tissues/organs for targeted delivery. The BBB is recognized as the major obstacle to the treatment of neurological disorders, as it hinders the delivery of many potentially important therapeutic and diagnostic substances to the CNS. These Nano-systems are showing a great potential as drug carriers to the brain, thanks to many advantages associated with their use, and are becoming an alternative to the present surgical and conventional methods. However, there is a need for further optimization of Nano system design and development of CNS therapeutics with enhanced activity and improved BBB permeability.
According to the various mechanisms of drug transport across BBB by Nano carriers, only penetration of drug-carrier conjugates into the brain parenchyma via transcytosis or endocytosis exhibit suitability for all types of brain drugs. Therefore, to obtain a practical delivery platform for brain drugs, it is suggested here that the development of a versatile delivery platform should focus on their capability as well as efficiency to be transcytosis or endocytosis.
Lastly, to achieve the minimal effective drug concentration in brain parenchyma while the maximal safety drug concentration is not exceeded in other organs, the drug transport efficiency across the BBB by Nano carriers should be elevated. From this viewpoint, it is necessary to explore new approaches facilitating endocytosis of Nano carriers by the brain capillary endothelial cells, although, in the brain parenchyma the efficiency of drug accumulation is not only determined by drug uploading and release profile, but also by crossing BBB, which are relatively easy to be engineered.
- Kermani F (1999) CNS Drugs and CNS Markets: A Strategic Guide to CNS Disorders, Markets and Therapies Available online at: http://www. Europe-anpharmaceutical.com
- Baker SK, Chen ZL Norris EH, Revenko AS, MacLeod AR, et al. (2018) Blood-derived plasminogen drives brain inflammation and plaque deposition in a mouse model of Alzheimer's disease. Proc Natl Acad Sci USA 115(41): 9687-9696.
- Modi G, Pillay V, Choonara YE, Ann NY (2010) Advances in the treatment of neurodegenerative disorders employing nanotechnology. Ann N Y Acad Sci 1184: 154-172.
- Simonato M, Bennett J, Boulis NM, Goins MG, Gray SJ, et al. (2013) Progress in gene therapy for neurological disorders. Nat Rev Neurol 9: 277-291.
- Itoh H, Pant H, Seno M (2013) Nano particles for brain drug delivery. 20 March 2013;2013.
- Barbu E, Molnar E, Tsibouklis J, Gorecki DC (2009) The potential for nanoparticle-based drug delivery to the brain: Overcoming the blood-brain barrier. Expert Opin Drug Deliv 6: 1-13.
- Masserini M (2013) Nanoparticles for brain drug delivery. Biochemistry 2013: e238428.16.
- McCarthy DJ, Malhotra M, O’Mahony AM, Cryan JF, O'Driscoll CM (2015) Nanoparticles and the blood-brain barrier: Advancing from in vitro models towards therapeutic significance. Pharm Res 32: 1161-1185.
- Partridge WM (2003) Blood-brain barrier drug targeting: The future of brain drug development. Mol Interv 3: 90-105.
- Kumar DK, Choi SH, Washicosky KJ, Eimer WA, Tucker S, et al. (2016) Amyloid-beta peptide protests against microbial infection in mouse and worm models of Alzheimer's disease. Sci Trans Med 8(340):340ra72.
- Majerova P, Garruto RM, Kovac A (2018) Cerebrovascular inflammation is associated with tau pathology in Guam Parkinsonism dementia. J Neural Trans Vienna 125(7): 1013-1025.
- Dimov N, Kastner E, Husain M, Perrie Y, Szita N (2017) Formation and purification of tailored liposomes for drug delivery using a module based micro continuous flow system. Sci Rep 7(1): 12045.
- Kaur M, Singh G, Khanna K, Kaur N (2015) Nanotechnology: A review. In Proceedings of the Second National Conference on Advances in Manufacturing Systems, S B S State Technical Campus, Ferozpur, India, pp: 23-24.
- Dong X (2018) Current strategies for brain drug delivery. Ranostics 8: 1481-1493.
- Abou el Ela AESF, El Khatib MM, Salem-Bekhit MM (2017) Design, characterization microbiological evaluation of micro-emulsion-based gel of Grisofulvin for topical delivery system. Bio-interfaces Appl Chem 7: 2277-2285.
- Fonseca-Santos B, Gremião MPD, Chorilli M (2015) Nanotechnology-based drug delivery systems for the treatment of Alzheimer’s disease. Int J Nano Med 10: 4981-5003.
- Shatzmiller S, Lapidot I, Zats G (2016) Blood brain barrier crossing for therapeutic and diagnostic agents. SM J Neurol Discord Stroke 2: 1012.
- Begley DJ (2004) Delivery of therapeutic agents to the central nervous system: the problems and the possibilities. Pharmacol Ther 104(1): 29-45.
- Begley DJ (2003) Understanding and circumventing the blood‐brain barrier. Acta Pediatrica 92: 83-91.
- Omidi Y, Barar J (2012) Impacts of blood-brain barrier in drug delivery and targeting of brain tumors. Bio Impacts 2(1): 5.
- Jörg K (2001) Nano particulate systems for brain delivery of Adv Drug Deliv Rev 47(1): 65-81.
- Jörg K (2013) Drug delivery to the central nervous system by polymeric nanoparticles: What do we know? Adv Drug Deliv Rev 71: 2-14.
- Lo EH, Singhal AB, Torchilin VP, Abbott NJ (2001) Drug delivery to damaged brain. Brain Res Rev 38(1-2): 140-148.
- Parboosing R, Maguire GEM, Govender P, Kruger HG (2012) Nanotechnology and the Treatment of HIV Infection. Viruses 4: 488-520.
- Wen CJ, Zhang LW, Al-Suwayeh SA, Yen T-C, Fang J-Y (2012) Theranostic liposomes loaded with quantum dots and Apo morphine for brain targeting and bio imaging. Int J Nanomed 7: 1599-1611.
- Chen H, Tang L, Qin Y, Yin Y, Tang J, et al. (2010) Lactoferrin-modified procationic liposomes as a novel drug carrier for brain delivery. Eur J Pharm Sci 40: 94-102.
- Wong HI, Wu XY, Bendayan R (2012) Nanotechnological advances for the delivery of CNS therapeutics. Adv. Drug Deliv Rev 64(7): 686-700.
- Lasic DD (1993) Liposomes: From Physics to Applications, Elsevier Science Publishers Raven Press: Amsterdam, the Netherlands.
- Tokes ZA, St Peteri AK, Todd JA (1980) Availability of liposome content to the nervous system. Liposomes and the blood-brain barrier. Brain Res 188: 282-286.
- Lee HJ, Engelhard B, Lesley J, Bickel U, Pardridge WM (2000) Targeting rat anti-mouse transferrin receptor monoclonal antibodies through blood-brain barrier in mouse. J Pharmacol Exp Ther 292(3): 1048-1052.
- Amjad MW, Amin MC, Katas H, Butt AM (2012) Doxorubicin-loaded cholic acid-polyethyleneimine micelles for targeted delivery of antitumor drugs: synthesis, characterization, and evaluation of their in vitro cytotoxicity. Nano scale Res Lett 7: 687.
- Kabanov AV, Alakhov VY (2009) Pluronic® block copolymers in drug delivery: From micellar nanocontainers to biological response modifiers. Crit Rev Ther Drug Carrier Syst 19(1): 1-72.
- Muthu MS, Rajesh CV, Mishra A, Singh S (2009) Stimulus-responsive targeted nano micelles for effective cancer therapy. Nanomedicine 4(6): 657-667.
- Bohr A, Water J, Beck-Broichsitter M, Yang M (2015) Nano embedded microparticles for stabilization and delivery of drug-loaded nanoparticles. Curr Pharm Design 21(40): 5829-5844.
- Bolhassani A, Javanzad S, Saleh T, Hashemi M, Aghasadeghi MR, et al. (2014) Polymeric nanoparticles: Potent vectors for vaccine delivery targeting cancer and infectious diseases. Hum Vaccin Immunother 10(2): 321-332.
- Teixidó M, Giralt E (2008) The role of peptides in blood‐brain barrier nanotechnology. J Pept Sci 14(2): 163-173.
- Domb AJ, Amselem S (1999) Antibiotic Delivery Systems for the Treatment of Chronic Bone Infections, John Wiley & Sons Ltd, Chi Chester, UK.
- Calvo P, Gouritin B, Chacun H, Desmaele D, Angelo D, et al. (2001) Long-Circulating PEGylated Polycyanoacrylate Nanoparticles as New Drug Carrier for Brain Delivery. Pharm Res 18: 1157-1166.
- Calvo P, Gouritin B, Villarroya H, Eclancher F, Giannavola C, et al. (2002) Quantification and localization of PEGylated polycyanoacrylate nanoparticles in brain and spinal cord during experimental allergic encephalomyelitis in the rat. Eur J Neuro Sci 15: 1317-1326.
- Wissing SA, Kayser O, Muller RH (2004) Solid lipid nanoparticles for parenteral drug delivery. Adv Drug Deliv Rev 561: 1257-1272.
- Muller RH, Mehnert E, Lucks JS, Schwarz C, Muhlen AZ, et al. (1995) Medication vehicles of solid lipid particles (Solid Lipid Nanospheres-SLN). Eur J Pharm Bio Pharm 41: 62.
- Barbara R, Belletti D, Pederzoli F, Masoni M, Keller J, et al. (2017) Novel curcumin loaded nanoparticles engineered for blood-brain barrier crossing and able to disrupt a beta aggregates. Int J Pharm 526: 413-424.
- Malinovskaya Y, Melnikov P, Baklaushev V, Gabashvili A, Osipova N, et al. (2017) Delivery of doxorubicin-loaded plga nanoparticles into u87 human glioblastoma cells. Int J Pharm 524: 77-90.
- Mondal J, Patra M, Panigrahi AK, Khuda-Bukhsh AR (2018) Improved drug carriage and protective potential against Cisplatin-induced toxicity using Boldine-loaded PLGA nanoparticles. J Ayurveda Integer Med 11(1): 24-36.
- Mallipeddi R, Rohan LC (2003) Progress in antiretroviral drug delivery using nanotechnology. J Nano Med 5: 533-547.
- Dhanikula RS, Hammady T, Hildgen P (2009) On the mechanism and dynamics of uptake and permeation of polyether-copolyester Dendrimer across an in vitro blood-brain barrier model. J Pharm Sci 98: 3748-3760.
- Bullen HA, Hemmer R, Haskamp A, Cason C, Wall S, et al. (2011) Evaluation of biotinylated PAMAM Dendrimer toxicity in models of the blood brain barrier: A biophysical and cellular approach. J Biomaterial Nanobiotechnol 2: 485-493.
- Wong HL, Chattopadhyay N, Wu XY, Bendayan R (2010) Nanotechnology applications for improved delivery of antiretroviral drugs to the brain. Adv Drug Deliv Rev 62: 503-517.
- Silva AM, Almeida MI, Teixeira JH, Maia AF, Calin GA, et al. (2017) Dendrite cell-derived extracellular vesicles mediated mesenchyme stem/ stoma cell recruitment. Sci Rep 7(1): 1667.
- Shtam TA, Kovalev RA, Varfolomeeva EY Makarov EM, Kil, et al. (2013) Exosomes are natural carriers of exogenous SiRNA to human cells in vitro. Cell Common Signal 11: 88.
- Koppers-lalic D, Hogenboom MM, Middeldrop JM, Pegtel DM (2013) Virus-Modified exosomes for targeted RNA delivery; A new approach in nanomedicine. Adv Drug Deliv Rev 65(3): 348-356.
- Ghaderi S, Ramesh B, Seifalian AM (2011) Fluorescence nanoparticle ‘‘quantum dots’’ as drug delivery system and their toxicity: A review. J Drug Target 19: 475-486.
- Gao X, Chen J, Chen J, Wu B, Chen H, et al. (2008) Quantum dots bearing lectin functionalized nanoparticle as a platform for in vivo brain imaging. Bioconjug Chem 19: 2189-2195.
- Santra S, Yang H, Stanley JT, Holloway PH, Moudgil BM, et al. (2005) Rapid and effective labeling of brain tissue using TAT-conjugated CdS: Mn/ZnS quantum dots. Chem Commun 2005: 3144-3146.
- Santra S, Yang H, Holloway PH, Stanley JT, Mericle RA (2005) Synthesis of water-dispersible fluorescent, radio-opaque, and paramagnetic CdS: Mn/ZnS quantum dots: A multifunctional probe for bio imaging. J Am Chem Soc 127: 1656-1657.
- Ganta S, Deshpande D, Korde A (2010) A review of multifunctional Nano emulsions systems to overcome oral and CNS drug delivery barriers. Mol Mem Biol 27: 260-273
- Calderón-Goercke M, Loricera J, Aldasoro V, Castañeda S, Villa I, et al. (2019) Tocilizumab in giant cell arteritis. Observational, open-label multicenter study of 134 patients in clinical practice. In Seminars in arthritis and rheumatism 2019 Aug 1. WB Saunders. 49(1): 126-135.
- Jaggi N, Rodrigues C, Rosenthal VD, Todi SK, Shah S, et al. (2013) Impact of an international nosocomial infection control consortium multidimensional approach on central line-associated bloodstream infection rates in adult intensive care units in eight cities in India. International Journal of Infectious Diseases 17(12): e1218-e1224.
- Silva AC, Kumar A, Wild W, Ferreira D, Santos D (2012) Long-term stability, biocompatibility and oral delivery potential of risperidone-loaded solid lipid nanoparticles. Int J Pharm 436(1-2): 798-805.
- Qian C, Decker EA, Xiao H, McClements DJ (2012) Nano emulsion delivery systems: Influence of carrier oil on β-carotene bio accessibility. Food Chem 135(3): 1440-1447.
- Marcato PD, Durán N (2008) New aspects of Nano pharmaceutical delivery systems. J Nano Sci Nanotechnol 8(5): 2216-2229.
- Kulkarni SA, Feng SS (2013) Effects of particle size and surface modification on cellular uptake and bio distribution of polymeric nanoparticles for drug delivery. Pharm Res 30(10): 2512-2522.
- Subramani K, Mehta M (2018) Chapter 19- Nano diagnostics in microbiology and dentistry. In Emerging Nanotechnologies in Dentistry, 2nd; Subramani, K., Ahmed, W., Eds.; William Andrew Publishing: Norwich, NY, USA pp: 391-419.
- Kafa H, Wang JT, Rubio N, Veneer K, Anderson G, et al. (2015) The interaction of carbon nanotubes with an in vitro blood-brain barrier model and mouse brain in vivo. Biomaterials 53: 437-452.
- Seok GE, Lu LT (2008) Invention of polysaccharide-based nanoparticles for enhancing drug permeability across the blood brain barrier. NSTI-Nanotech 2: 379-381.
- Azadia A, Hamidib M, Khoshayandc MR, Amini M, Rouini MR, et al. (2012) Preparation and optimization of surface-treated methotrexate-loaded nanogels intended for brain delivery. Carbohydr Polym 90: 462-471.
- Liu Y, Gao S, Hu Z, Gao C, Zong K, et al. (2010) Continental and oceanic crust recycling-induced melt-peridotite interactions in the Trans-North China Orogen: U-Pb dating, Hf isotopes and trace elements in zircons from mantle xenoliths. J Petrol 51(1-2): 537-571.
- Ljubimova JY, Sun T, Mashouf L, Ljubimov AV, Israel LL, et al. (2017) Covalent Nano delivery systems for selective imaging and treatment of brain tumors. Adv Drug Deliv Rev 113: 177-200.
- Sharma G, Sharma AR, Lee SS, Bhattacharya M, Nam JS, et al. (2019) Advances in nanocarriers enabled brain targeted drug delivery across blood brain barrier. Int J Pharm 559: 360-372.
- Naqvi S, Panghal A, Flora SJ (2020) Nanotechnology: A promising approach for delivery of neuroprotective drugs. Front Neurosci 14: 494.





