25
Views & Citations10
Likes & Shares
Patients with human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) frequently present alterations in lipid metabolism due to infection with HIV itself, including elevated serum concentrations of triglycerides and low levels of total cholesterol [6].
Micronutrients are trace elements and vitamins obtained from our diet that are essential to sustain life and optimal physiological function [7]. Deficiencies affect over 2billion people and are largely associated with malnutrition or poor diet [8,7]. Many micronutrients are necessary to elicit an effective immune response to viral infections but are also utilized by viruses such as the hepatitis C virus (HCV) and hepatitis B virus (HBV) to propagate infections [9]. Essential micronutrients are involved in many metabolic pathways in the liver, such as enzymatic functions and protein synthesis, oxidative damage and anti-oxidant defense, immunological competence, interferon therapy response regulations, and alterations of the virus genomes [10,11]. Reactive oxygen species (ROS) have also been implicated in a number of hepatic pathologies in exacerbating liver diseases [12,13]. The oxidant production associated with immune reactions against viral hepatitis leads to the formation of hepatocellular carcinoma (HCC) [14]. Therefore, the changes in micronutrients and their demolishing effects against oxidative stress are factors for viral hepatitis pathogenesis.
Micronutrients which comprise of essential trace elements (e.g. Zinc, Copper and Selenium) and vitamins (e.g. Vitamin A, C and E) are nutrients needed in minute specific quantities in the body which play leading roles in the production of enzymes, hormones and other substances, helping to regulate growth activity, essential in wound healing, development and the functioning of the immune and reproductive system, maintenance of the integrity of skin and mucous membranes, which also function as a barrier to bacteria and viruses and protection against lipid peroxidation among several others [15-18]. Most micronutrients are not generated in the body but are derived from food intake [19-21]. Deficiencies of these micronutrients may result in: fatigue, depression, and widespread abnormalities in connective tissue, such as inflamed gingivae, petechiae, perifollicular hemorrhages, impaired wound healing, coiled hairs, hyperkeratosis and bleeding into body cavities [22]. It may also lead to damage of cell membrane and leakage of cell contents to the extracellular fluid compartment, cardiac or skeletal myopathies, neuropathies, and liver necrosis, muscle and neurological problems [23]. Still, deficiencies of some micronutrients have been associated with impaired immunological functions [24,25]. For instance, zinc deficiency has been reported to decrease lymphocyte concentrations, copper deficiency reduced cytokine response, while selenium deficiency negatively impacted on proper functioning of the neutrophils and T-lymphocytes [26,27].
The highly active antiretroviral therapy (HAART) is currently the therapy of choice for HIV-infected patients. However, despite remarkable viral replication suppression and immune response resto-ration, adverse drug reactions appear to be a major drawback to the success story of long-term HAART use in many patients [28,29]. HAART regimen have been implicated in increase generation of chemically reactive species in circulation, possibly by producing more oxidized metabolites derived from the interaction between ROS and infected cell biomolecules [30]. This suggests that the HIV-1 infection alone or in combination with the introduction of antiretroviral (ARV)/HAART may induce oxidative stress and further augment HIV-1 pathogenesis [30,31]. Measurement of oxidative/nitrosative stress molecules could therefore function as a potential surveillance parameter in addition to CD4+ T cell count for estimation of progression of infection and development of adverse drug reactions. Findings from this study may provide the much desired improved therapeutic and management effects on individuals living with HIV/AIDS. However, we hereby perform this research to assess some micronutrients of HIV positive subjects visiting Central Hospital, Uromi, Edo State, Edo State.
MATERIALS AND METHODS
Area of Study
This study was carried out in Uromi, the administrative headquarters of Esan North-East Local Government Area of Edo State, Nigeria. The area proper is situated at 6.70 North latitude, 6.330 East longitude. Uromi is the largest and most populated area in Esanland, settled by two waves of people, made up of villages divided into three groups recognized as: Okhiode (Eguare, Egbele, Onewa, Utako, Unuwazi, Arue and Isua, Uje Oror), Obiruan (Ebhoyoman, Efadion, Ekhue, Ubierumun, Eror, Obeidu, Uwalor, Idumoza, Ivue) and Obiyuan (Ukoni, Amedeokhian, Awo). Uromi has a population of 108,608 (NPCN, 2012), majority of which are civil servants, traders, businessmen/women, transporters, farmers, teachers/lecturers and students by occupation. The samples were examined in the Research Diagnostic Laboratory, of the Department of Medical Laboratory Science, College of Medicine, Ambrose Alli University, Ekpoma.
Population of the Study
The subjects in this study comprised of HIV positive volunteers aged between 18 to 50 years attending Central Hospital Uromi, Edo State, Nigeria. A total of one hundred (100) samples were recruited for this study. The sample size (N) is calculated from the formula below using prevalence from previous studies. The study was conducted on 100 subjects comprising of fifty (50) apparently healthy subjects (controls) and fifty (50) HIV positive subjects (test samples).
Research Design
This study was designed as a prospective and cross-sectional study to assess some micronutrients of HIV positive subjects visiting Central Hospital, Uromi, Edo State, Edo State, Nigeria. The results of the micronutrients obtained for the HIV positive subjects obtained was compared with that of the control. Also, a complete record of medical history (age, gender and other important medical information) was obtained for each subject from the patient’s medical records. This study was carried out within three (3) months from June 2022 to August, 2022. Furthermore, in order to ensure HIV status validity, both confirmed and negative cases were re-tested by the researcher with HIV test strips using standard Laboratory procedures. It is on the basis of this validity that HIV positive and control subjects were selected and grouped for the study. The overall results of the study obtained was compared with the control using appropriate statistical methods.
Ethical Considerations
Ethical permission for this study was obtained from the Health Research Ethics Committee of Ambrose Ali University, Ekpoma, Edo State, Nigeria. Also, informed consent was sought and obtained from the patients prior to the collection of samples for this study. The purpose of the study was exhaustively explained to the patients and assured of the confidentiality of the information obtained from them.
Only already diagnosed and confirmed HIV positive subjects and apparently healthy subjects was recruited as control subjects for this study. This study specifically includes only subjects between 18-55 years who gave consent for the study. Individuals who are not within the age range of 18-55 years whose HIV status has not been confirmed were excluded. Control subjects who show any sign of visible ailments, did not give consent and have any underlying illness such as diabetes, cardiovascular, sickle cell and renal diseases were also excluded from this study.
Sample Collection
Five milliliters (5mls) of venous blood sample were collected from each subject from the ante-cubital vein using sterile disposable syringe. The blood samples were immediately placed in a labelled lithium heparin and EDTA containers for both subjects and control individuals for the estimation of micronutrients. The blood was spun for 10 minutes at 5000 rpm. The serum was separated from the red cells using a dry clean Pasteur pipette into dry clean plain specimen containers which was labelled corresponding to the initial blood samples containers. The serum was then stored at -20°C pending the analysis of the samples.
Sample Analysis
HIV Serology Test: HIV serostatus was determined according to center for disease and prevention (CDC-UMD). HIV rapid testing serial algorithm II guideline (WHO, 1997). Determine HIV-½ kit, an immunochromatographic technique, was the kit used. Positive results by “Determine HIVI/II” kit (Alere Medical Co., Ltd., Chiba, Japan) was confirmed with “Unigold HIVI/II” test kit (Trinity Biotech Plc., Bray, Ireland) while negative results by “Determine HIVI/II” ended testing. Discordant results are repeated and finally tested with a tie-breaker kit-stat pak. Final results were considered positive or negative on the basis of tie-breaker result.
Determine HIVI/II Test
Principle: Alere Determine HIVI/II is an immunochromatographic test for the qualitative detection of antibodies to HIV I/II. Sample is added to the sample pad. As the sample migrates through the conjugate pad, it reconstitutes and mixes with the selenium colloid-antigen conjugate. This mixture continues to migrate through the solid phase to the immobilized recombinant antigens and synthetic peptides at the patient window site [32]. If antibodies to HIV -1 and/or HIVII are present in the sample, the antibodies bind to the antigen-selenium colloid and to the antigen at the patient window, forming a red line at the patient window site. If antibodies to HIVI/II and /or HIVII are absent, the antigen-selenium colloid flows past the patient window, and no red line is formed at the patient window site. To ensure assay validity, a procedural control bar is incorporated in the assay device.
Procedure
- The protective foil cover was removed from each test.
- 50µL of sample was applied to the sample pad
- It was allowed to stand for 15 minutes and the result was read.
Interpretation of Results
POSITIVE (Two Bar): Red bars appear in both the control window (labeled “Control”) and the patient window (labeled “Patient”) of the strip. Any visible red bar in the patient window should be interpreted as positive.
NEGATIVE (No Bar): One red bar appears in the control window of the strip (labeled “control”) and no red bar appears in the patient window of the strip (labeled “Patient”).
UNIGOLD HIVI/II TEST
Principle: HIV is a rapid immunoassay based on the immunochromatographic sandwich principle. Recombinant proteins representing the immunodominant regions of the envelope proteins of HIVI/II and HIVII, glycoprotein gp41, gp120 (HIVI/II) and glycoprotein gp36 (HIVII) respectively, are immobilized at the test region of the nitrocellulose strip. These proteins are also linked to colloidal gold and impregnated below the test region of the device. A narrow band of the nitrocellulose membrane is also sensitized as a control region [32].
Procedure
- The devices were laid on a clean flat surface and it was labeled with appropriate subject information / ID.
- The disposable pipette included in the kit was filled with sample avoiding air bubbles and the pipette was not reused.
- The pipette was held vertically over the sample port, the bulb was squeeze and two drops of plasma was discharged or applied unto the sample pad.
- The sample was allowed to fully absorbed.
- The test results were read after 10 min.
Interpretation of Results
Reactive Test Result: Two pink/red lines of any intensity in the device window, the first adjacent to letter “T” (test) and the second adjacent to “C” (control). This indicates a Reactive result that is interpreted as Preliminary Positive for antibodies to HIV.
Non-Reactive Test Result: A pink/red line of any intensity adjacent to the letter “C” (control), but no pink/red line adjacent to “T” (test). This indicates a Non-Reactive result that is interpreted as Negative for antibodies to HIV.
Serum Selenium (μg/dL): Serum Selenium was estimated by the Ferrozine method of White & Flashka, (1973)
Principle: Serum is treated with buffer to prevent precipitation of proteins and to provide an acid medium to dissociate ferric-transferrin complex and to reduce ferric to ferrous ion. Addition of color reagent forms a deeply colored ferrozine-iron complex with maximum absorbance at 562nm.
Reference values: Adults: Male: 60-150μg/dL; Female: 50-130μg/dL
Serum Copper (μg/dL): Serum Copper was measured using Diethyldithiocarbamate method (Practical Clinical Biochemistry, 1980) of Eden & Green, 1940; Ventura & King, 1951.
Principle: After releasing copper from protein by hydrochloric acid, the proteins were precipitated by trichloroacetic acid, and the copper extracted into mixture of amyl alcohol and ether as a golden yellow colored complex with sodium diethyldithiocarbamate for colorimetric determination at 440nm.
Reference interval: Birth to 6 months: 20-70μg/dL; 6 months-6 years: 90-190μg/dL; 6 years-12 years: 80-160μg/dL; Adult Male: 70-140μg /dL; Adult Female: 80-155μg /dL.
Serum Calcium Estimation: Calcium was estimated using the method described by Robertson & Mashall, (1979).
Principle: Calcium ion reacts with O-cresophthalene-complex ono in an alkaline medium to form a purple colored complex. The absorbance of this complex is proportional to the calcium concentration in the sample.
Statistical Analysis
The Mean and standard deviation of the results obtained was calculated. ANOVA (LSD) was used for the analysis using SPSS package version 21. Values with p< 0.05 shall be considered statistically significant in this study.
RESULTS
The results of this study are presented in this chapter and comparisons were made on some micronutrients (Zn, Ca, Cu and Se) between the control (HIV-negative) and HIV-positive subjects visiting Central Hospital, Uromi, Edo State. Socio demographic profiles and the history of the subjects were also presented.
Table 1 revealed the socio-demographic characteristics of the study population. The subjects were categorized into four age groups; 23-30 years; 31-40 years; 41-50 years; 51 years and above. The result for age showed that majority of the subjects were within the age range of 51 years and above; 19(38.0%), this was followed by 41-50years, 18(36.0%), 31-40 years; 10(20.0%) and 23-30 years; 3(6.0%). The results also showed that 13(26.0%) of the subjects were male and 37(74.0%) of the subjects were female. As regards to the Period of been on ART, majority of the subjects were with the range of new-5years; 22(44.0%), this was followed by 11-15years; 14(28.0%), 6-10years; 13(26.0) and 16 years and above; 1(2.0%). The Age (Mean ±SD) were (3.06±0.91).
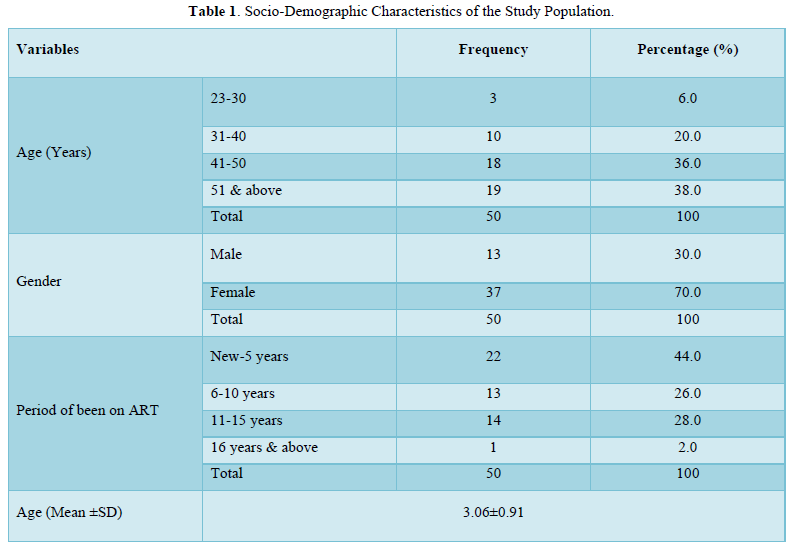
The results in Table 2 showed the comparison of zinc, copper, calcium and selenium levels HIV positive subjects and HIV negative subjects. The results showed that zinc levels were not significantly lower (p>0.05) in HIV positive subjects (0.79±0.48mg/l) when compared with that of the negative subjects (0.91±0.53mg/l). Also, copper levels were significantly not lower (p>0.05) in positive subjects (0.84±0.41mg/l) when compared with the negative subjects (0.87±0.36 mg/l). On the contrary, calcium levels were significantly lower (p<0.05) in positive subjects (80.12±14.71mg/l) when compared with negative subjects (89.03±14.82mg/l). Furthermore, selenium levels were also significantly lower (p<0.05) in positive subjects (0.08±0.02mg/l) when compared with the negative subjects (0.10±0.02mg/l).
The results in Table 3 showed the comparison of zinc, copper, calcium and selenium levels of male HIV positive subjects and male HIV negative subjects. The results showed that zinc levels were not significantly lower (p>0.05) in HIV positive subjects (0.83±0.57mg/l) when compared with that of the negative subjects (0.94±0.56mg/l). Copper levels were significantly not higher (p>0.05) in positive subjects (0.90±0.51mg/l) when compared with the negative subjects (0.85±0.38mg/l). On the contrary, calcium levels were significantly lower (p<0.05) in positive subjects (77.65±14.37mg/l) when compared with negative subjects (93.64±14.03mg/l). Furthermore, selenium levels were not significantly higher (p>0.05) in positive subjects (0.89±0.03mg/l) when compared with the negative subjects (0.09±0.03mg/l).

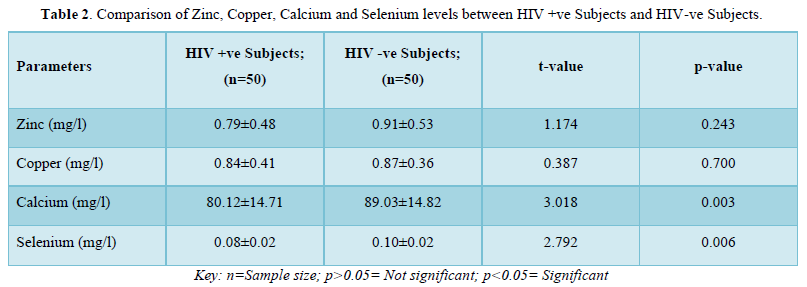
The results in Table 4 showed the comparison of zinc, copper, calcium and selenium levels of female HIV positive subjects and female HIV negative subjects. The results showed that zinc levels were not significantly lower (p>0.05) in HIV positive subjects (0.78±0.45mg/l) when compared with that of the negative subjects (0.89±0.51mg/l). Copper levels were also significantly not lower (p>0.05) in positive subjects (0.82±0.38mg/l) when compared with the negative subjects (0.89±0.35mg/l). Calcium levels were also not significantly lower (p>0.05) in positive subjects (80.99±14.92mg/l) when compared with negative subjects (86.66±14.87mg/l). On the contrary, selenium levels were significantly lower (p>0.05) in positive subjects (0.08±0.02mg/l) when compared with the negative subjects (0.10±0.02mg/l).
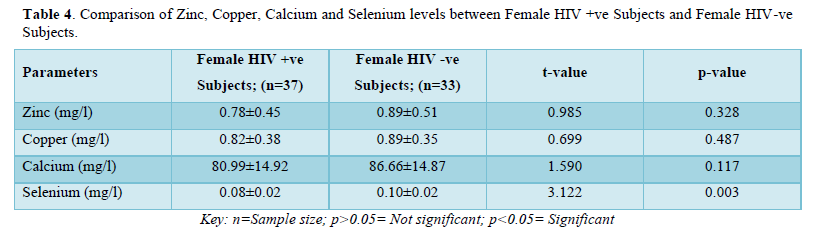
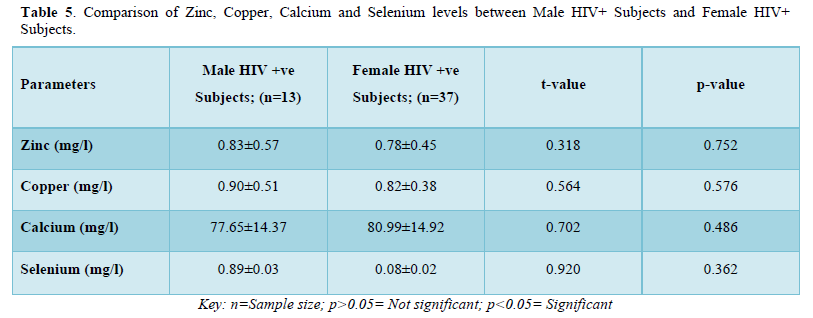
The results in Table 5 showed the comparison of zinc, copper, calcium and selenium levels between male HIV positive subjects and female HIV positive subjects. The results showed that zinc levels were not significantly higher (p>0.05) in male HIV positive subjects (0.83±0.57mg/l) when compared with that of female positive subjects (0.78±0.45mg/l). Copper levels were not significantly higher (p>0.05) in male positive subjects (0.90±0.51mg/l) when compared with the female positive subjects (0.82±0.38mg/l). Furthermore, calcium levels were not significantly lower (p>0.05) in male positive subjects (77.65±14.37mg/l) when compared with female positive subjects (80.99±14.92mg/l). Selenium levels were not significantly higher (p>0.05) in male positive subjects (0.89±0.03mg/l) when compared with the female positive subjects (0.08±0.02mg/l).
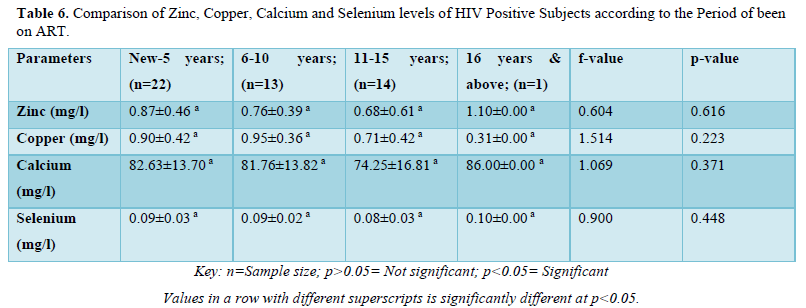
The results in Table 6 showed the comparison of zinc, copper, calcium and selenium levels of HIV positive subjects according to the period of been on ART. The results showed that zinc levels were not significantly higher (p>0.05) in subjects that have has been on Art within the range of new-5years (0.87±0.46mg/l) when compared with 6-10years (0.76±0.39mg/l), 11-15years (0.68±0.61mg/l) and 16years and above (1.10±0.00mg/l). Copper levels were not significantly higher (p>0.05) in subjects within the range of 6-10years (0.95±0.36mg/l) when compared with new-5years (0.90±0.42mg/l), 11-15years (0.71±0.42mg/l) and 16years and above (0.31±0.00mg/l). Calcium levels were not significantly higher (p>0.05) in subjects that have has been on Art within the range of 16years and above (86.00±0.00mg/l) when compared with new-5years (82.63±13.70mg/l), 6-10years (81.76±13.82mg/l) and 11-15years (74.25±16.81mg/l). Selenium levels were not significantly higher (p>0.05) in subjects that have has been on Art within the range of 16years and above (0.10±0.00mg/l) when compared with new-5years (0.09±0.03mg/l), 6-10years (0.09±0.02mg/l) and 11-15years (0.08±0.03mg/l).
The results in Table 7 showed the comparison of zinc, copper, calcium and selenium levels of HIV positive subjects according to age. The results showed that zinc levels were not significantly higher (p>0.05) in subjects within the age range of 31-40years (0.97±0.43mg/l) when compared with 23-30years (0.95±0.25mg/l), 51years and above (0.80±0.49 mg/l) and 41-50years (0.66±0.52mg/l). Copper levels were significantly higher (p<0.05) in subjects within the age range of 23-30years (1.03±0.64mg/l) when compared with 51years and above (1.01±0.44mg/l), 31-40years (0.99±0.28mg/l) and 41-50years (0.57±0.26mg/l). Calcium levels were also significantly higher (p<0.05) in subjects within the age range of 23-30years (94.47±5.65mg/l) when compared with 31-40years (87.84±6.64mg/l), 51years and above (80.18±16.70mg/l) and 41-50years (73.38±13.48mg/l). Furthermore, selenium levels were also significantly higher (p<0.05) in subjects within the age range of 23-30years (0.09±0.04mg/l) when compared with 31-40years (0.09±0.02mg/l), 51years and above (0.09±0.02mg/l) and 41-50years (0.06±0.02mg/l).

DISCUSSION
The results from this study showed that 13(26.0%) of the subjects were male and 37(74.0%) of the subjects were female but this is probably predicated on the fact that the HIV positive subjects were gotten by consecutive clinic recruitment from the HIV clinic in Uromi where females have been shown to seek care more than males. This female preponderance in HIV infection is consistent with a study in Zaria, Kaduna State, Nigeria [33] and the publication by UNAIDS 2002 at Geneva. This result is however contradictory to Giri [34] study in Northern India and Cheong [35] in Kuala Lumpur, Malaysia. However suffice to state that reasons have been adduced for the relatively higher proportion of women with HIV infection such as polygamy, low literacy level, practice of female genital mutilation (FGM), other harmful cultural practices, early age at first sex of adolescent girls, many sex partners as seen in commercial sex workers and the fact that, a higher proportion of men (30%) than women (19%) in less developed countries have comprehensive knowledge of HIV and how to avoid transmission [36]. The sex distribution of the HIV negative subjects would be inconsequential because the HIV negative subjects were from friends, neighbors, and other willing volunteers with already ascertained HIV status.
In this study, the subjects were categorized into four age groups; 23-30years; 31-40years; 41-50years; 51years and above. The result for age showed that majority of the subjects were within the age range of 51years and above 19(38.0%), this was followed by 41-50years 18(36.0%), 31-40years 10(20.0%) and 23-30years 3(6.0%), this agrees with similar work done by HIV surveillance team of the United states of America Center for Diseases and Control (CDC, 2009) and other researchers (NYSC, 2007) in Abuja, Nigeria. The high prevalence noticed in 31-40- and 23-30-years age groups is consistent with some studies in Nigeria [33,37]. These age groups are also characterized by social vices such as, unwanted pregnancy, unsafe abortions, drug use and sexually transmitted infections [33].
Serum micronutrient levels are used to characterize micronutrients deficiencies which are influenced by factors such as gender, time of the day of measurement, acute infection, liver disease, experimental error and recent intake [38]. There may be interaction between micronutrients and concomitant antiretroviral drug therapy making generalization of findings to diverse population difficult [38]. The results of micronutrient treatment studies are difficult to interpret due to various study designs, doses, lengths of follow-up time, and study outcomes. Serum micronutrient levels are used to characterize micronutrient deficiencies, but they may not be a true reflection of nutritional status. Micronutrient levels are influenced by factors such as gender, time of day of measurement, acute infection, liver disease, technical parameters, and recent intake. There may be interaction between micronutrients, and between micronutrients and concomitant antiretroviral drug treatment therapy, making generalization of findings to diverse populations difficult [39]. The outcome of this study shows that micronutrients and electrolytes deficiencies exist in HIV infected individuals.
The results from this study showed that zinc levels were lower (p>0.05) in HIV positive subjects (0.79±0.48 mg/l) when compared with that of the negative subjects (0.91±0.53 mg/l) but was not statistically significant which was similar to values seen in research by Nwegbu [40] and Gadallah, (2000), Zinc is required by HIV to form enzymes such as viral integrase, which plays a key role in its replication [41]. Zn is essential for maintaining immune system function, and in the presence of oxidative stress, without a corresponding increase in supply Zn level there would result a deficiency. This possibly explains the reduced plasma levels of Zn in the HIV positive subjects. Decreased plasma zinc concentrations have been associated with advanced disease and increased mortality in HIV patients [42]. Also, in this study, the results showed that zinc levels were higher (p>0.05) in male HIV positive subjects (0.83±0.57 mg/l) when compared with that of female positive subjects (0.78±0.45 mg/l) but was not statistically significant. This is in contrast to results obtained by Milbury and Richer [43] though the higher number of female HIV subjects in our study could have skewed our findings in this regard.
Selenium levels were also significantly lower (p<0.05) in HIV positive subjects (0.08±0.02 mg/l) when compared with the negative subjects (0.10±0.02 mg/l). This is consistent with the findings of Eley [44], Bobat [41] Ogunro [45] Fawzi [46] Kiremidjian-Schumacher [47] and Zhang [48] but in contrast with the study conducted by Nwegbu [40]and Khalili [49]. The reduction in Selenium level may be caused by several factors such as latent state induced by the virus, malabsorption, altered metabolism, gut infection, altered gut barrier function and the hypermetabolic state [50- 52]. It has also been suggested that a possible cause of selenium depletion among HIV positive Subjects is the utilization of selenium by HIV-l virus to produce its own selenoenzymes [53-55]. Recent research indicates that HIV may be capable of incorporating host selenium into viral selenoproteins that have glutathione peroxidase activity [48] but as an integral component of glutathione peroxidase and thioredoxin reductase, selenium plays an important role in decreasing oxidative stress in HIV-infected cells possibly suppressing the rate of HIV replication [47]. Therefore the fact that “decreasing plasma selenium concentrations in HIV-infected individuals are sensitive markers of disease progression and severity”, even before malnutrition becomes a factor [46] and Low levels of plasma selenium been associated with a significantly increased risk of death from HIV infection, would indicate our study caught HIV subjects that were in the state of early course (low severity)of the infection thus their high level of Se or as a result of the supplementary micronutrient taken by the HIV subjects. Selenium levels were higher (p>0.05) in male positive subjects (0.89±0.03 mg/l) when compared with the female positive subjects (0.08±0.02 mg/l) but was not statistically significant. Selenium deficiency, more than any other nutrient, has been documented to correlate with progression and mortality of HIV [56]. Selenium is needed for the proper functioning of the immune system, and appears to be a key nutrient in counteracting the development of virulence and inhibiting HIV progression to AIDS [57]. When taken as a supplement, selenium modulates the cellular response to oxidative stress, inducing a faster restoration of the endogenous antioxidative defense system against the production of reactive oxygen species [58].
Copper levels were lower (p>0.05) in HIV positive subjects (0.84±0.41 mg/l) when compared with the negative subjects (0.87±0.36 mg/l) but was not statistically significant. This is in contrast with the findings of previous studies done by Lawal [59] and Nwegbu [40] who both reported a significant increase in the mean plasma copper concentration of HIV infected Subjects when compared with control group. Copper as a compound is required for immune complex formation, blood and coagulation factors formation. In fact, it is a major micronutrient required by the body in HIV infection [60].
Calcium levels were significantly lower (p<0.05) in HIV positive subjects (80.12±14.71mg/l) when compared with negative subjects (89.03±14.82mg/l). Calcium has been shown to reduce diarrhea in HIV-positive/AIDS patients (Hammond, 2004) [61]. Calcium levels were not significantly lower (p>0.05) in male positive subjects (77.65±14.37 mg/l) when compared with female positive subjects (80.99±14.92mg/l). Copper levels were not significantly higher (p>0.05) in male positive subjects (0.90±0.51mg/l) when compared with the female positive subjects (0.82±0.38mg/l). In addition, poor dietary intakes, poor absorption and diarrhea which is common in HIV infection, may also have contributed to the reduced levels of micronutrients and electrolytes in the HIV subjects [62]. Also, the recycling mechanism of biologically active vitamin E through ascorbate has been reported to be impaired in HIV patients [59,63].
Low or deficient serum concentrations of micronutrients in HIV infected patients has been individually associated with either low CD+T-cell counts, advanced HIV-related diseases, increased disease progression or mortality [16,25,64,65]. Possible mechanisms as earlier posited include increased intracellular oxidative stress which is responsible for the production of reactive oxygen species (ROS) such as superoxide anion, hydroxyl radical, and hydrogen peroxide. Excessive production of ROS has been associated with increased stimulation of HIV replication, increased pro-oxidant effect of tumor necrosis factor (TNF) and accelerated development of Immunodeficiency contributing to the increased morbidity, more rapid disease progression, and the higher mortality seen in HIV-infected patients with micronutrient deficiencies [29,66,67]. Furthermore, the mean±SD HAART serum zinc, copper, calcium and selenium levels were observed to be lower than the control in this study. This contradicts the work of Batterham [68] who observed no significant difference in serum selenium between those on HAART and the control group.
This study has shown that HIV patients suffer significant oxidative stress. There is impairment of the antioxidant defense mechanism in HIV patients, and this is further affected by drug therapy used in the management of the condition. It may prove useful for treatment of HIV infection to include antioxidant therapy. It remains unclear if supplementation with micronutrients has any measurable impact on the clinical course of HIV disease. Further research is needed to elucidate clinical benefit of supplementation in different clinical settings, and with different micronutrients. Still, there is considerable collective evidence that nutritional compromise adversely affects the course of HIV disease. It has also been shown that micronutrient supplements may alleviate symptoms, delay progress to AIDS, reduce mortality, accelerate growth in children, improve birth outcomes, and reduce maternal mortality.
The decrease in micronutrients that accompanies HIV infection suggests a potentially important role of nutritional supplementation and good nutrition in the proper management of HIV/AIDS. Ideally, every individual diagnosed with HIV infection should be screened for micronutrient deficiencies, and a nutritionist consulted, especially in later stages of disease. However, this is not always feasible. Multivitamin and trace element supplements should be considered for all persons with HIV infection.
CONCLUSION
In conclusion, our study findings highlight the need for assessment of micronutrient status of relevant elements with proven immunological functions in HIV infected people and correction of deficiencies where observed. It is also important to assess and document any nutritional supplements being taken by these patients, by their respective physicians. This will assist in proper evaluation and interpretation of research findings involving micronutrients in this group of patients. This latter observation was a limitation in this study and further studies taking this into cognizance as well as with larger sample sizes are needed to clarify the myriad and sometimes contrasting findings in this area of research.
We therefore recommend that HIV infected Patients should be investigated and treated for micronutrients, if present, to reduce the morbidity and mortality associated with HIV infection. Close monitoring of patients before and during HAART is very critical to aid in evaluating drug combinations and implementation of dose modifications when necessary. The information obtained in this study will therefore help health workers to emphasize on the use of HAART in their campaign for HIV management and encourage HIV patients in making themselves available to be treated especially at the early stage of infection.
ETHICAL APPROVAL AND CONSENT TO PARTICIPATE
The approval for this study was given by the ethical committee of Health Research Ethics Committee of Ambrose Ali University, Ekpoma, Edo State, Nigeria. Informed consent was obtained from each participant prior to specimen collection.
AVAILABILITY OF DATA AND MATERIALS
The authors declare consent for all available data present in this study.
FUNDING
This research did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors.
AUTHORS’ CONTRIBUTIONS
- Conception and design the work/idea: Omolumen, L.E. and Iyevhobu, K.O.
- Collect data/obtaining results: Airhomwanbor, K.O. and Abaku, P.O.
- Manuscript writing: Iyevhobu, K.O., and Abaku, P.O.
- Analysis and interpretation of data: Omolumen, B.A., Ugege, C. and Usiobeigbe, O.S.
- Critical revision of the manuscript: Omokpo, V.O., Adeji, A.J. and Obohwemu, K.O.
- Statistical advice: Ikede, R.E., Ayo, A. and Oikerhe, E.G.
- Final research review: Animasaun, O.P., Bisiriyu, A.H. and Omisakin, I.A.
COMPETING INTERESTS
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the management of Central Hospital, Uromi, Edo State, Edo State, Nigeria for creating the enabling environment for this study. Thanks to all the Laboratory and technical staff of St Kenny Research Consult, Ekpoma, Edo State, Nigeria for their excellent assistance and for providing medical writing/editorial support in accordance with Good Publication Practice (GPP3) guidelines.
- WHO/UNAIDS (2009) Global summary of the HIV/AIDS epidemic. December 2009. Accessed on: June 01, 2022. Available online at: www.unaids.org
- Federal Ministry of Health Nigeria (FMOH) (2006) 2005 National HIV/Syphilis sero-prevalence sentinel survey among pregnant women attending antenatal clinics: technical report, April. Abuja, Federal Ministry of Health. Accessed on: July 01, 2021.
- Park K (2019) Surface infection. Park’ textbook of preventive and social medicine 25th India: Bhanot publishers; pp: 371.
- UNAIDS (2019) Fact sheet: Global HIV & AIDS statistics, 2019. Accessed on: June 01, 2022. Available online at: http://www.unaids.org
- The Joint united Nations Programme on HIV/AIDS (UNAIDS) (2018) Gap Report 2018; UNAIDS Fact Sheet. UNAIDS /JC2656 ISBN 978-92-9253-062-4
- Grunfeld C, Kotler DP, Hamadeh R, Tierney A, Wang J, et al. (2009) Hypertriglyceridemia in the acquired immunodeficiency syndrome. Am J Med 86: 27-31.
- Bailey RL, West KP Jr, Black RE (2015) The epidemiology of global micronutrient deficiencies. Ann Nutr Metab 66: 22-33.
- Howson CP, Kennedy ET, Horwitz A (1998) Prevention of Micronutrient Deficiencies: Tools for Policymakers and Public Health Workers; Committee on Micronutrient Deficiencies, Board on International Health, Food and Nutrition Board: Washington, DC, USA.
- Rashed MN (2011) The role of trace elements on hepatitis virus infections: A review. J Trace Elem Med Biol 25: 181-187.
- Bhaskaram P (2002) Micronutrient malnutrition, infection, and immunity: An overview. Nutr Rev 60: S40-S45.
- Ozcelik D, Ozaras R, Gurel Z, Uzun H, Aydin S (2003) Copper mediated oxidative stress in rat liver. Biol Trace Elem Res 96: 209-215.
- Toubi E, Kessel A, Goldstein L, Slobodin G, Sabo E, et al. (2001) Enhanced peripheral T-cell apoptosis in chronic hepatitis C virus infection: Association with liver disease severity. J Hepatol 35: 774-780.
- Loguercio C, Federico A (2003) Oxidative stress in viral and alcoholic hepatitis. Free Rad Biol Med 34: 1-10.
- Jain SK, Pemberton PW, Smith A, McMahon RF, Burrows PC, et al. (2012) Oxidative stress in chronic hepatitis C: Not just a feature of late stage disease. J Hepatol 36: 805-811.
- Berger MM, Shenkin A (2006) Update on clinical micronutrient supplementation studies in the critically ill. Curr Opin Clin Nutr Metab Care 9(6): 711-716.
- Akinola FF, Akinjinmi AA, Oguntibeju OO (2012) Effect of Combined Antiretroviral Therapy on Selected Trace Elements and CD4 T-cell Count in HIV-Positive Persons in an African Setting. J AIDS Clin Res 3: 185.
- Akinboro AO, Onayemi O, Ayodele OE, Mejiuni AD, Atiba AS (2013) The impacts of first line highly active antiretroviral therapy on serum selenium, CD4 count and body mass index: A cross-sectional and short prospective study. Pan Afr Med J 15: 97.
- Attah RA, Attah CJ, Muhammed MJ (2016) Effect of Micronutrients on HIV infected Patients Receiving Highly Active Antiretroviral Therapy and its Implication on markers of renal disease. South Asian J Med 1(2): 22-28.
- Evans P, Halliwell B (2001) Micronutrients: Oxidant/antioxidant status. Br J Nutr 85(2): S67-S74.
- Singh M (2004) Role of micronutrients for physical growth and mental development. Indian J Pediatr 71(1): 59-62.
- Fledler JL, Macdonald B (2009) A strategic approach to the unfinished fortification agenda: feasibility, costs, and cost-effectiveness analysis of fortification programs in 48 countries. Food Nutr Bull 30(4): 283- 311.
- Eipper B, Milgram SL, Husten EJ, Yun H, Mains RE (1993) Peptidylglycine alpha amidiating monooxygenae: A multifunctional protein with catalytic, processing, and routing domains. Protein Sci 2(4): 489-497.
- Berger MM, Chioléro RL (2003) Key vitamins and trace elements in the critically ill. Nestle Nutr Workshop Ser Clin Performance Programme 8: 99-111.
- Drain PK, Kupka R, Mugusi F, Fawzi WW (2007) Micronutrients in HIV-positive persons receiving highly active antiretroviral therapy. Am J Clin Nutr 85(2): 333-345.
- Allavena C, Dousset B, May T, Dubois F, Canton P (2015) Relationship of trace element, immunological markers, and HIV1 infection progression. Biol Trace Elem Res 47(1-3): 133-138.
- Fraker PJ, King LE, Laakko T, Vollmer TL (2000) The dynamic link between the integrity of the immune system and zinc status. J Nutr 130: 1399S-1406S.
- Ferencík M, Ebringer L (2003) Modulatory effects of selenium and zinc on the immune system. Folia Microbiol 48(3): 417-426.
- Fisher JD, Fisher WA, Cornman DH, Amico RK, Bryan A, et al. (2006) Clinician-delivered intervention during routine clinical care reduces unprotected sexual behavior among HIV-infected patients. J Acquir Immune Defic Syndr 41(1): 44-52.
- Mandas A, Lorio EL, Congiu MG, Balestrieri C, Mereu A (2009) Oxidative imbalance in HIV-1 infected patients treated with antiretroviral therapy. J Biomed Biotechnol 7: 1-7.
- Hulgan T, Donahue JP, Hawkins C, Unutmaz D, D'Aquila RT, et al. (2003) Implications of T-cell P-glycoprotein activity during HIV-1 infection and its therapy. J Acquir Immune Defic Syndr 34(2): 119-126.
- Sharma A, Slaughter A, Jena N, Feng L, Kessl JJ, et al. (2014) A new class of multimerization selective inhibitors of HIV-1 integrase. PLoS Pathol 10(5): e1004171.
- Behne D, Kyriakopoulos A (2001) Mammalian selenium-containing proteins. Ann Rev Nutr 21: 453-473.
- Laah JG (2003) The prevalence of HIV/AIDS in Zaria, Kaduna State. J Popul Assoc Nigeria 3(1): 95-101.
- Giri TK, Wali JP, Meena HS, Pande I, Uppal S, et al. (1995) Socio demographic characteristics of HIV infection in Northern India. J Comm Dis 127(1): 1-9.
- Cheong I, Lim A, Lee C, Ibrahim Z, Sarvanathan K (1997) Epidemiology and clinical characteristics of HIV-infected patients in Kuala Lumpur. Med J Malaysia 52: 313-317.
- Bremner J, Haub C, Lee M, Mather M, Zuehlke E (2009) World population highlights: Key findings from PRB’s world population data sheet. Popul Bull 64(3): 2-12.
- Mamman M (2003) Gender HIV-infection and AIDS - Related deaths in sub-Saharan Africa. J Popul Assoc Nigeria 3(1): 79-94.
- Neera S (2002) A clinical review of micronutrients in HIV infection. Int J Assoc Physic AIDS Care (1): 1-12.
- Brod-Miller C (1990) Hypomagnesemia (Hmg) in acquired immune deficiency syndrome (AIDS). J Am Sociol Nephrol 1: 329-331.
- Nwegbu MM, Egua MO, Ogwu OS (2015) Comparative study of plasma zinc and selenium levels amongst Human Immunodeficiency Virus (HIV) positive and negative subjects. Afr J Food Sci Technol 6(8): 253-258.
- Bobat R, Coovadia H, Stephen C (2005) Safety and efficacy of zinc supplementation for children with HIV-1 infection in South Africa: A randomized double-blind placebo-controlled trial. Lancet 366(9500): 1862-1867.
- Mocchegiani E, Muzzioli M (2000) Therapeutic application of zinc in human immunodeficiency virus against opportunistic infections. J Nutr 130(5): 1424-1431.
- Milbury PE, Richer AC (2008) Understanding the Antioxidant Controversy: Scrutinizing the "fountain of Youth". Greenwood Publishing Group pp: 99.
- Eley BS, Sive AA, Abelse L, Kossew G, Copper G (2002) Growth and micronutrient disturbance in stable, HIV-infected children in Cape Town. Ann Tropl Pediatr 22(1): 19-23.
- Ogunro PS, Ogungbamigbe TO, Elemie PO, Egbewale BE, Adewole TA (2006) Plasma selenium concentration and glutatione peroxidase activity in HIV/AIDS infected patients: A correlation with the disease progression. Nigerian Postgraduate Med J 13(1): 1-5.
- Fawzi W (2003) Micronutrients and HIV type-1 disease progression among adults and children. Clin Infect Dis 37 (2): 112-116.
- Kiremidjian-Schumacher L, Roy M, Glickman R (2000) Selenium and immunocompetence in patients with head and neck cancer. Biol Trace Elem Res 73(2): 97-111.
- Zhang W, Ramanathan CS, Nadimpalli RG, Bhat AA, Cox AG, et al. (1999) Selenium-dependent glutathione peroxidase modules encoded by RNA viruses. Biol Trace Elem Res 70(2): 97-116.
- Khalili H, Ian K, Bryan M (2011) Department of Pharmacotherapy, School of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran.
- Grunfeld C, Kotler DP (1992) Pathophysiology of the AIDS wasting syndrome. AIDS Clin Rev 7: 191-224.
- Steinhart CR (2001) HIV-associated wasting in the era of HAART: A practice-based approach to diagnosis and treatment. AIDS Res 11: 557-569.
- Tang AM, Forrester J, Spiegelman D (2002) Weight loss and survival in HIV-positive patients in the era of highly active antiretroviral therapy. J Acquir Immune Defic Syndr 31(2): 230-236.
- Shisler JL, Senkevich TG, Berry MJ, Moss B (1998) Ultraviolet-induced cell death blocked by a selenoprotein from a human dermatotropic poxvirus. Science 279(5347): 102-105.
- Xu XM, Carlson BA, Grimm TA (2002) Rhesus monkey simian immunodeficiency virus infection as a model for assessing the role of selenium in AIDS. J Acquir Immune Defic Syndr 31(5): 453-463.
- Okunade K, Olowoselu OF, Osanyin GE, Olabode SJ, Akanmu SA (2018) Selenium deficiency and pregnancy outcome I pregnant women with HIV in Lagos, Nigeria. Int J Gynecol Obstet 142(2): 207-213.
- Baum MK, Shor-Posner G, Lai S (2017) High risk of HIV-related mortality is associated with selenium deficiency. J Acquir Immune Defic Syndr Retrovirol 15: 370-374.
- Rayman MP (2000) The importance of selenium to human health. Lancet 356(9225): 233-241.
- Jozanov-Stankov O, Demajo M, Djujić I, Mandić M (1998) Selenium intake as a modulator of responsiveness to oxidative stress. J Environ Pathol Toxicol Oncol 17(3-4): 251-257.
- Lawal S, Bilbis DB, Idowu YS, Mansur L, Chibueze HN (2010) Serum levels of antioxidant vitamins and minerals elements of Human Immunodeficiency Virus Positive Subjects in Sokoto, Nigeria. Ann Afr Med 9(4): 235-239.
- Percival SS (1998) Copper and immunity. Am J Clin Nutr 67: 1064S-1068S.
- Hammond KA (2004) Dietary and Clinical Assessment, vol. 372, WB Saunders, Philadelphia, Pa, USA, 11th edition, pp: 20-34.
- Dworkin BM, Wormser GP, Axelrod F, Pierre N, Schwarz E (1990) Dietary intake in patients with acquired immunodeficiency syndrome (AIDS), patients with AIDS-related complex, and serologically positive human immunodeficiency virus patients: Correlations with nutritional status. J Parenter Enteral Nutr 14(6): 605-609.
- Tang AM, Graham NMH, Semba RD, Saah AJ (2017) Association between serum vitamin A and E levels and HIV-1 disease progression. J Acquir Immune Defic Syndr 11(5): 613-620.
- Beach RS, Mantero-Atienza E, Shor-Posner G (2012) Specific nutrient abnormalities in asymptomatic HIV-1 infection. J Acquir Immune Defic Syndr 6: 701-708.
- Tang AM, Graham NM, Kirby AJ, McCall LD, Willett WC (2013) Dietary micronutrient intake and risk of progression to acquired immunodeficiency syndrome (AIDS) in human immunodeficiency virus type 1 (HIV-1)-infected homosexual men. Am J Epidemiol 138(11): 937-951.
- Schwartz KB (1996) Oxidative stress during viral infection: A review. Free Rad Biol Med 21(5): 641-649.
- Allard JP, Aghdassi E, Chau J, Salit I, Walmsley S (1998) Oxidative stress and plasma antioxidant micronutrients in humans with HIV infection. Am J Clin Nutr 67(1): 143-147.
- Batterham M, Gold J, Naidoo D, Lux O, Sadler S (2001) A preliminary open label dose comparison using an antioxidant regimen to determine the effect on viral load and oxidative stress in men with HIV/AIDS. Eur J Clin Nutr 55(2): 107-114.
- Forrester J, Sztam K (2011) Micronutrients in HIV/AIDS: Is there evidence to change the WHO 2003 recommendations. American J Clinical Nutrition 94(6): 1683S-1689S.
- World Gazetteer. (2007) Population of Cities, news, divisions. Accessed on: May 23, 2022. Available online at: http://world gazetteer.com/ng.php

