Research Article
Chocolate-Coated Popcorn: A Healthy and Functional Snack
4614
Views & Citations3614
Likes & Shares
The possible improvement of the health benefits of popcorn via coating with chocolate was investigated. Free and bound polyphenols were extracted from plain popcorn (PP), chocolate (Ch) and chocolate-coated popcorn (CCP), and analysed separately. The total polyphenol content (TPC) was measured using Folin-Ciocalteu assay, and trolox equivalent antioxidant capacity (TEAC) was analysed using DPPH and ABTS methods. The extracted phenolic compounds were analysed using a HPLC technique. Ch showed significantly (P<0.05) the highest TPC of 1560.49 ± 21.84 mg gallic acid equivalent (GAE)/100 g dry weight (DW). Additionally, Ch revealed the largest TEAC of 2559.50 ± 69.1 and 2378.50 ± 108.0 mg Trolox equivalent (TE)/100g DW, measured by DPPH and ABTS, respectively. CCP had significantly (P<0.05) smaller quantities of TPC and TEAC compared with Ch. PP had greater amounts of bound polyphenol (BPP) while both Ch and CCP had significantly (P<0.05) more free polyphenols (FPP). The HPLC analysis indentified nine different phenolic compounds in CCP including gallic acid (131.88 ± 3.70 mg/g DW), vanillic acid (30.04 ± 1.20 µg/g DW), caffeic acid (34.37 ± 1.33 µg/g DW), trans-ferulic acid (4.80 ± 0.30 µg/g DW), p-coumaric acid (13.37 ± 0.24 µg/g DW), sinapic acid (9.51 ± 0.50 µg/g DW), (±)-catechin hydrate (500.17 ± 10.36 µg/g DW), (-)-epicatechin (356.38 ± 5.47 µg/g DW) and quercetin hydrate (75.39 ± 3.93 µg/g DW).
Keywords: Chocolate, Popcorn, Antioxidants, Antioxidants capacity, HPLC
PRACTICAL APPLICATION
Chocolate-coated popcorn (CCP) is anticipated to offer the consumers a healthier snack. The CCP will be a good souce of fibre from the popcorn itself as well as free polyphenols from the coating chocholate. Additionally, the CCP will provide the confectionary industry with the opportunity to manufacture this new type of healthy snack and generate more profit.
INTRODUCTION
Snacking is described as the intake of food and drinks in between main meals, with snack foods being a frequently consumed convenience food [1,2]. In Australia, 35% of daily energy source of school-going children are from snacks, while for adults, snacks contribute 28% of daily energy intake. Here, snacks were defined as any food intake which resulted in a minor peak of energy intake. Both children and adults consume snacks two to three times per day, apart from the three main meals. Although snack consumption decreases with age, snack consumption in adults has increased over the years [3]. Likewise, 35% of Australian’s daily energy intake came from discretionary foods. People consume snacks in an array of everyday life spaces and situations, often most associated with leisure-based and social activities, such as celebrations, watching TV, hiking or going to the movies. Young consumers use food and frequently snack, as a means to cultivate connections and maintain relationships.
Chocolate is an antioxidant-rich snack and has an extensive positive effect on health. Chocolate is known for its beneficial effects on the cardiovascular system, insulin sensitivity, immune system, central nervous system and psychological wellbeing [4-6]. According to the Australian Bureau of Statistics, 17% of the population consume chocolate and chocolate-based confectionary, and it is the most favoured choice of confectionary.
Chocolate is a product of cocoa, where the cocoa seeds from the tropical Theobroma cacao L. tree undergo various processes including fermentation, drying, roasting and grinding. Total polyphenol content and antioxidant capacity in chocolate depends on the proportion of cocoa liquor present, and corresponds to the non-fat cocoa solid (NFCS) content [5-8]. Dark chocolate contains one of the highest amounts of antioxidant [9], and is a major dietary source of flavonoids [10]. Cocoa bean processing also affects total polyphenol content. The longer the duration of bean fermentation, the higher the flavonoid concentration and the lengthier the roasting time, the lower the antioxidant capacity. However, the loss that occurs during the roasting stage can be compensated by the synthesis of Maillard reaction products such as melanoidins [11]. Dutching, or alkalization treatment neutralizes the natural acidity of cocoa, resulting in a milder taste and darker colour of cocoa [12,8]. Alkalization is known to reduce polyphenol content and alter the epicatechin to catechin ratio in dutched cocoa powder [12,13,8].
The major polyphenols in chocolate are the flavonoids, specifically procyanidins (58%), flavanols (catechins) (37%), and anthocyanins (4%). Flavanols are catechin or epicatechin monomers, procyanidins are oligomers of flavanols, while proanthocyanidins are polymers of flavanols.
Similarly, popcorn is a favourite snack for both adults and children. A sector trend analysis of the snack food industry in the United States found that popcorn had the greatest growth with a compound annual growth rate of 7.1% from the year 2011 to 2015 [14]. Popcorn, which is fibre-rich and low in calories, is considered an optimal snack choice and healthy when consumed on its own [15,16]. Popcorn, a whole grain snack, provides greater satiety than potato chips and is rich in nutrients [6]. Popcorn (Zea Mays Everta) differs from regular corn by its ability to expand to many times their original size [17]. The endosperms of popcorn kernels are surrounded by a layer of extremely hard shell called the pericarp [18]. When the kernels are subjected to heat, moisture within the endosperm or starch layer turn to gas. Due to the hard pericarp, the gas is trapped inside; allowing popcorns to expand [19] Popcorns are rich in fibre the fibre [17] in popcorn is found in the pericarp, with the major fibre being hemicelluloses. Australian Food, Supplement and Nutrient Database (AUSNUT) [20] reported popcorn as having between 5.2 to 16.5 g of dietary fibre per 100 g of popcorn, depending on the method of preparation.
It has been reported that main antioxidants present in microwave-popped yellow popcorn without oil were carotenoids (the highest) [16], followed by the polyphenol’s anthocyanins and phenolic acids. The main carotenoids in popcorn included xanthophylls, zeaxanthin and lutein, and the minor carotenoids were β-carotene, β-crptoxanthin and α-crptoxanthin [21,22]. Phenolic acids have also been detected in popcorn and represented by benzoic acid, vanillic acid, tannic acid, o-coumaric acid and caffeic acid [22]. However, the possible health effects of popcorn coated with chocolate has not been fully investigated. Consequently, this study aims to evaluate whether a combination of popcorn and chocolate in the form of chocolate-coated popcorn would yield a higher total polyphenol content and better antioxidant capacity compared with plain popcorn.
Experimental design
This study was performed using a split plot random design, where three trials were conducted at different times following exactly the same procedures. At least two measurements were repeated for each parameter within each trial (n=6). Results were reported as means followed by standard deviation.
Materials
Popping corn (Riviana Popping Corn Kernels) and baking chocolate (Cadbury Baking Chocolate Block, 70% Cocoa) were purchased from a local supermarket in Melbourne. Acetone, methanol, hydrochloric acid, acetic acid, dimethyl sulfoxide (DMSO), sodium carbonate and sodium hydroxide were purchased from the Bio-21 specialist store (Melbourne, Australia).Folin-Ciocalteu reagent (2M),2,2-Diphenyl-1-picrylhydrazyl (DPPH), potassium persulfate, 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), (±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox), gallic acid, (-)-epicatechin, vanillic acid, caffeic acid, quercetin hydrate, p-coumaric acid, (±)-catechin hydrate, sinapic acid and trans-ferulic acid were at least of analytical standard and purchased from Sigma-Aldrich Co, Castle Hill, New South Wales, Australia. HPLC-grade acetonitrile was purchased from Merck Millipore, Bayswater, Victoria, Australia.
METHODS
Preparation of chocolate-coated popcorn
Popcorn was popped in a microwave (X2-30ES, Whirlpool) under same standard conditions. Baking chocolate was melted in a water bath. Popcorn was stirred in the melted chocolate to ensure that each popcorn was equally covered with chocolate. Chocolate-coated popcorns (CCP) were then arranged onto a wire rack to allow any excess chocolate to drip off, and for the chocolate to cool and harden. All three samples: plain popcorn (PP), chocolate (Ch) and chocolate-coated popcorn (CCP) were freeze-dried for 48 hr (Dyna Vac FD3, USA). Moisture contents in the freeze-dried PP, CCP and Ch were 1.40%, 1.14% and 0.34%, respectively. Freeze-dried samples were then ground into powder using a coffee grinder (Sunbeam EM0405), passed through a 1.029 mm sieve, transferred into zip-lock plastic bags and stored in a dessicator until used.
Extraction of free polyphenols
Free polyphenols (FPP) extraction was performed following the methods [23]. Powdered samples (1.5 g each) were mixed with 15 mL extraction solvent consisting of acetone: methanol: water (2:2:1, v/v/v). The mixture was then placed in a shaking incubator (Labwit ZWYR-240, China) at 40 °C, 150 rpm for 1 hour and centrifuged (Allegra X-12R, Beckman Coulter) at 22 °C, 10,000 rpm for 10 minutes. Extraction was repeated two more times with 5 mL extraction solvent. All supernatants were collected and the extract was made up to 25 mL in a volumetric flask using methanol. The extract was stored at -20 °C until analysis. The remaining residue was used for the extraction of bound polyphenols.
Extraction of bound polyphenols
The residue remaining from FPP extraction was used to extarct and quantitate the bound polyphenols (BPP) contents. Known quantaty of the residue was quantitatively transferred and mixed with 25 mL methanol/HCl (97:3, v/v) and placed in a 100 °C water bath for 3 hr. The mixture was then centrifuged at 22 °C, 10,000 rpm for 10 minutes. The supernatant was collected and the residue was subjected to three additional washings with 3 mL methanol each time. The combined supernatants were rotary evaporated at 60 °C (Hei-VAP Rotary Evaporator, Heidolph), and the pH of the final concentrated extract was adjusted to ~5. The extract was brought to 10 mL in a volumetric flask using Milli-Q water and stored at -20 °C until analysis [23].
Determination of Total Polyphenol Content (TPC)
Total polyphenol content (TPC) was quantified using Folin-Ciocalteu assay following the methods with slight modifications. Briefly, FPP and BPP extracts were diluted with Milli-Q water, such that the diluted extract and reagent give an absorbance between 0.1 and 0.5 at 760 nm, which is within the absorbance range of gallic acid standard curve. Sample extracts (20 μL each) were mixed with 100 μL of Folin-Ciocalteau reagent (1/10) in microplate wells. Within 5 min of the addition, 80 μl of 7.5% (w/v) sodium carbonate solution was added to each well. The reaction mixture was then incubated in a dark at room temperature for 1 hr. Following incubation, the absorbance was read at 760 nm using a microplate reader (Multiskan GO, Thermo Scientific, Australia) at room temperature. A standard curve using gallic acid (0 - 0.12 mg/mL) was generated, andTPCs were calculated and expressed as mg gallic acid equivalence (GAE)/ 100 g sample dry weight (DW).
Determination of antioxidant capacity via the dpph assay
Antioxidant capacity was determined using Diphenyl-1-picrylhydrazyl (DPPH), following the method (24,25). Diluted sample extract (20 μL) was mixed with 180 μL DPPH (0.15 mM) in a microplate well. The reaction mixture was then incubated in the dark at room temperature for 40 minutes before measuring the absorbance at 515 nm. The scavenging actvity% was calculated using equation 1. A linear calibration curve was generated using 0.020 to 0.080 mg Trolox /mL to determine antioxidant capacity. Trolox equivalent antioxidant capacity (TEAC) of samples were expressed as mg Trolox equivalence (TE)/100 g sample DW.
Equation 1: Calculation for scavenging capacity
Scavenging capacity (%) =
Determination of antioxidant capacity via the ABTS Assay
Antioxidant capacity was measured using the 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assay by adopting the methods. ABTS working solution was prepared by diluting the radical (ABTS·+) solution with methanol, to approximately 0.85 absorbance at 734 nm. Sample extracts were diluted with methanol so that they produced between 20-60% inhibitions of ABTS radical solution, which is within the range of the Trolox standard curve generated. The sample extracts (25 µL each) were mixed with 250 µL of ABTS working solution in micro plate wells. The mixtures were incubated with shaking at room temperature for 7 minutes in the micro plate reader, before measuring the absorbance at 734 nm. The percentage inhibition was calculated using equation 1. A linear calibration curve was generated using 0.020 to 0.080 mg Trolox /mL to determine antioxidant capacity. Results were expressed as mg TE/100 g sample DW.
HPLC analysis of free polyphenols
Undiluted sample extracts and standards (1 mL) were filtered through a 0.45 µm nylon filter disk (Thermo Fisher) prior to injection into HPLC. Chromatographic separation was carried out following the methods of [26] HPLC analysis was carried out using a Waters 2690 Alliance HPLC separation module (Waters, Rydalmere NSW, Australia) and Waters 2998 Photodiode Array (PDA) detector. The mobile phase consisted of solvent A: Milli-Q water/acetonitrile/acetic acid (89:9:2, v/v/v) and solvent B: acetonitrile/MilliQ water (80:20, v/v). The gradient used was: 0-10 min, 0% B; 10-30 min, 0-40% B; 30-37 min, 40-100% B; 37-42 min, 100% B. Samples and standards (20 µL) were injected into the HPLC system. The column was equilibrated for 5 minutes before and in between injections. Column temperature was set at 37 °C.
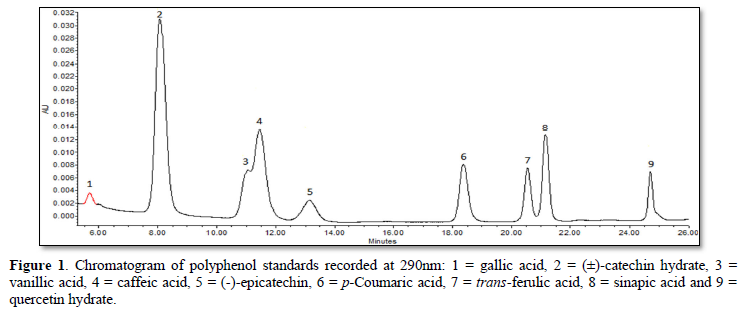
Separation of polyphenols was performed with a Gemini C18 silica 250 × 4.6 mm, 5 µm column (Phenomenex Inc., Lane Cove West, NSW, Australia) attached to a guard column, and detected at 280 nm and 290 nm. A total of nine external standards were used for peak identification by comparing the retention times. Those standards included gallic acid, (±)-catechin hydrate, vanillic acid, caffeic acid, (-)-epicatechin, p-coumaric acid, trans-ferulic acid, sinapic acid and quercetin hydrate. Three different concentrations of each standard were prepared in dimethyl sulfoxide (DMSO) in order to generate a linear standard curve.
Vanillic acid (VA) and caffeic acid (Caff) were not well separated, and the external standards of both compounds had approximately the same retention time. Consequently, mixtures of vanillic acid and caffeic acid were prepared at different ratios: (a) VA: Caff (1:1, v/v) and (b) (3:1, v/v). Both standards had concentrations of 0.01 mg/mL, and the injection volume was 20 µL. With the VA: Caff (1:1) mixture, 0.1 µg vanillic acid and 0.1 µg caffeic acid was injected; with VA: Caff (3:1) mixture, 0.15 µg vanillic acid and 0.05 µg caffeic acid was injected. VA: Caff (1:1) mixture was first injected, followed by VA: Caff (3:1) mixture. The area of peak 1 increased from 32% (Appendix 1, peak 1a) to 64% (peak 1b); area of peak 2 reduced from 68% (peak 2a) to 36% (peak 2b). Thus, it was deduced that peak 1 represented vanillic acid and peak 2 represented caffeic acid, and that vanillic acid eluted before caffeic acid.
Statistical analysis
Statistical analyses of all the results were carried out using one-way analysis of variance (ANOVA) to assess for any differences between the samples. The means obtained were separated by Tukey pairwise comparison test at 95% confidence level using MINITAB 17 statistical software (Minitab® Statistical Software, 2010). All data were presented as means ± SD (n=6). Pearson’s coefficient (R2) was also identified for all calibration curves. Pearson’s correlation (r) was calculated at P
RESULTS AND DISCUSSION
Total polyphenol content
The free polyphenols (FPP) and bound polyphenols (BPP) fractions were analysed separately, and the calculated sum was reported as total polyphenol content (TPC). Both chocolate (Ch) and chocolate-coated popcorn (CCP) had significantly (P<0.05) greater amounts of FPP than plain popcorn (PP) alone. However, FPP content in Ch (802.89 ± 23.98 mg GAE/100g DW) was significantly (P<0.05) higher than those in CCP (639.74 ± 15.05 mg GAE/100g DW) and PP (87.01 ± 1.35 mg GAE/100g DW) (Table 1). The same trend was observed for BPP and TPC fractions. Total polyphenol content (TPC) in all three samples varied significantly (P<0.05) from 1560.49 ± 21.84 mg GAE/100g DW to 1103.32± 13.58 mg GAE/100g DW and 505.25 ± 10.05 mg GAE/100g DW in Ch, CCP and PP, respectively (Table 1). It was clear that TPC in plain popcorn was attributed mainly to BPP (418.24 ± 10.87 mg GAE/100g DW) in comparison with FPP (87.01 ± 1.35 mg GAE/100g DW). On the contrary, both CCP and Ch had significantly (P<0.05) higher FPP than BPP content. The FPP content detected in CCP and Ch were 639.74 ± 15.05 mg GAE/100g DW and 802.89 ± 23.98 mg GAE/100g DW, respectively (Table 1 and Figure 1).
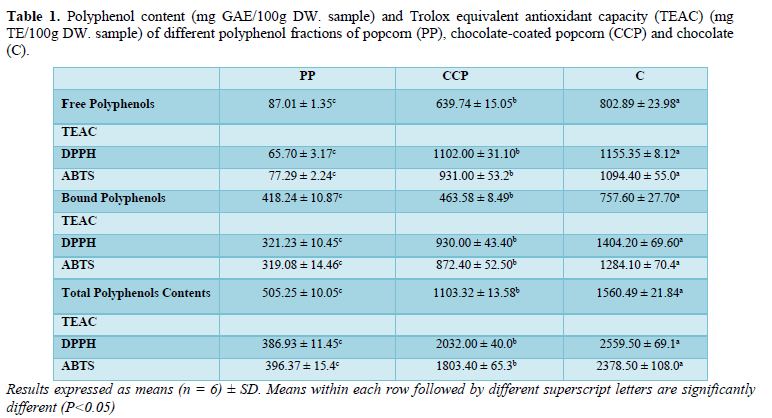
The amounts of FPP (87.01 ± 1.35 mg GAE/100g DW) and TPC (505 ± 10.05 mg GAE/100g DW) in PP were greater than those values reported in the literature for yellow popcorn popped without oil. A FPP content of 7 mg GAE/100g DW while TPC of 131 mg GAE/100g DW. Furthermore, the detected FPP content in PP was higher than in popcorn kernel (37 mg GAE/100g DW). On the other hand, TPC in PP reported in this study was lower than those in popcorn kernels of 539 mg GAE/100g DW and 797 mg GAE /100g DW, respectively. Such variations could be attributed to many factors, including, popcorn variety and method of preparation, extraction and analysis.
The BPP represent the main polyphenols in whole grains, and the calculated value from results in Table 1 showed that BPP represented approximately 83% of TPC in plain popcorn. This percentage BPP content was within the range obtained for corn kernels (62-85%) [27,7], but lower than the 95% for popcorn kernels. Unfortunately, the literature on BPP Pin popped popcorn is lacking, and most studies only focused on FPP.
The FPP content measured in baking chocolate (803 mg GAE/100g DW equivalent to 800 mg GAE/100g FW) was within the values in dark chocolate(800 - 840 mg GAE/100g FW) [28], but smaller than those, 1816 mg GAE/100g FW [29]. The TPC (1560 mg GAE/100g DW equivalent to 1555 mg GAE/100g FW) (Table 1) was within the range (1176 to 2970 mg GAE/100g FW) [30], but smaller than the 5146 mg GAE/100g FW [31].
It has been estimated that in baking chocolate, about half of the polyphenols existed as BPP, mostly as condensed tannins [32,34]. Similarly, polyphenol content in dark chocolate consisted of 38% FPPs and 62% BPP; while in milk chocolate, FPP made up 53% and BPP made up 47% of total polyphenols [35]. It should be noted that polyphenol composition (FPP and BPP) detected in this current investigation did not agree with the findings of dark chocolate, but was similar to that of milk chocolate. This could possiblydue to the fact that measured BPP content using a different method and expressed the results as condensed tannin equivalents. Additionally, the presence of milk influenced polyphenol content. Milk solids are one of the raw materials used in the production of our baking chocolate sample, which could possibly cause the composition to be similar to that of milk chocolate. Another contributing factor to variations in reported result could be the methods of polyphenols extraction. Previous studies investigated the phenolic content in yellow popcorn and baking chocolate using aqueous organic solutions of methanol, ethanol and/or acetone [28,22]. These extraction conditions can only liberate the FPP, while the BPP remains in the residue [36,37]. consequently, the values reported by these authors underestimated TPC and were smaller than the amount reported in the current study.
Antioxidant Capacity
Trolox equivalent antioxidant capacity (TEAC) for free polyphenols (FPP) and bound polyphenols (BPP) were performed separately, using DPPH and ABTS assays. The TEAC in total polyphenols (TPP) were then calculated from the individual data of FPP and BPP fractions. Both DPPH and ABTS assays showed similar results, in which the FPP fraction in Ch and CCP had significantly (P<0.05) higher antioxidant capacity than in PP. Additionally, TEAC of FPP in Ch was significantly higher (P<0.05) than those in CCP. The same trend was observed for BPP. Similar to the previous findings of polyphenol contents, the TEAC in Ch was significantly (P<0.05) the highest, followed by those in CCP, and in PP in descending order for both FPP and BPP fractions (Table 1).
The TEAC detected in the TPP fraction of PP [386.93 ± 11.45 (DPPH method) and 396. 37 ± 15.4 mg TE/100g DW (ABTS)] (Table 1) were within the range (128 – 96825 mg TE/100g DW) in popcorn kernels, and slightly smaller than those (400 - 501 mg TE/100g DW). The high TEAC in PP can be attributed to the significantly (P<0.05) large quantities of BPP (83%) compared with FPP (17%).
The TEACs of FPP measured in chocolate and on fresh weight bases for easy comparison with the literature values (1091 and 1151 mg TE/100g FW, using ABTS and DPPH, respictevely) were higher than the range in dark chocolate (881-961 mg TE/100g FW), and lower than (1972 to 3751 mg TE/100g FW). Additionally, the TEAC in the TPC fraction of chocolate (2370-2551 mg TE/100g FW) varied from literature values reported in dark and baking chocolates. The TEAC in Ch was greater than the amounts reported (3.78 – 621.22 and 2000 mg TE/100g FW, respectively), and smaller than those observed (3475-7357 TE/100g FW) [38,39].
The antioxidant capacities of FPPs and BPPs in Ch were not in accordance with their respective polyphenol contents. The FPP content (802.89 mg GAE/100 g DW) was larger than that in BPP (757.60 mg GAE/100 g DW). While, the TEAC in the BPP fraction (1404.2 mg TE/100g DW) was greater than in FPP (1155.35 mg TE/100 g DW) of chocolate. This inconsistency could be attributed to the presence of glycoside-bound polyphenols in BPP extracts, which have highly reactive scavenging compounds, causing BPP to have a higher antioxidant capacity than FPP [40-43].
On the contrary to the resuslts of TAEC in Ch, data in Table 1 revelaed good corellation between the reported amounts of free and bound polyphenols and the TEAC in CCP. The larger contente of FPP (639.74 mg GAE/ 100 g Dw) yielded greater amount of TEAC (1102,00 mg TE/ 100 g DW). These resulsts are in agrement with the pervious suggestion that glycoside-bound polyphenols in BPP extracts contributed more to the antioxidant capacity. Consequently, the significantly the smaller amount of BPP (463.58 mg GAE/ 100 g Dw) in comparison with FPP (639.74 mg GAE/ 100 g DW) generated less TEAC in CCP.
The differences in measured antioxidant capacity using DPPH and ABTS methods could be due to the differences in reactivity of antioxidants in the analysed samples with the radicals in ABTS and DPPH [44].
Phenolic compounds in free polyphenol fractions
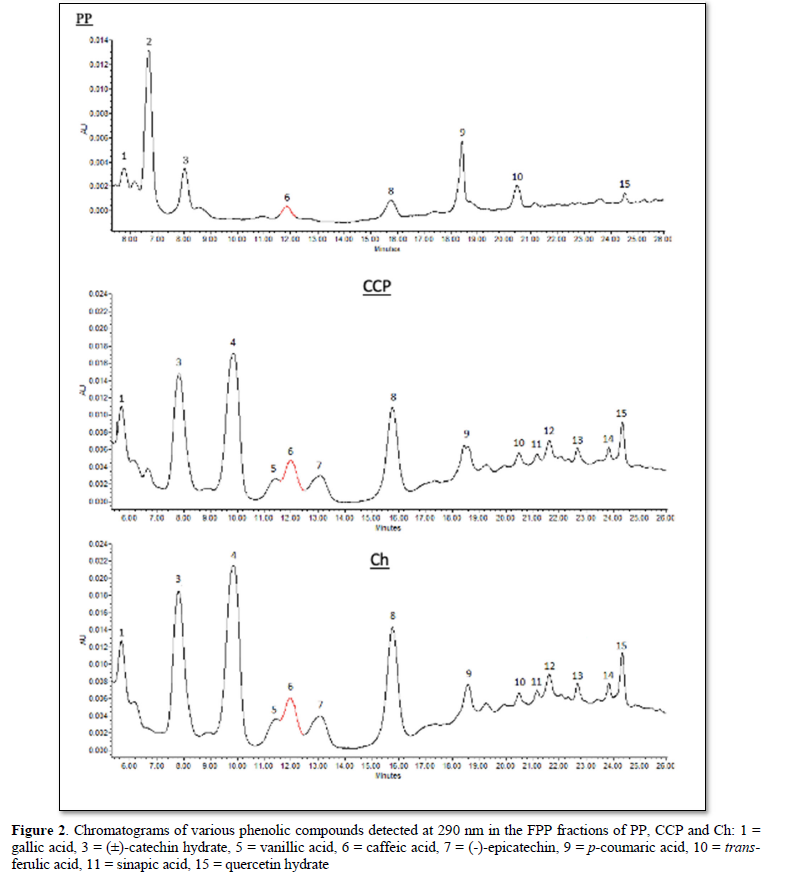
Using a mobile phase of methanol and acetic acid, two flavanol compounds (catechin, epicatechin), one flavonol (quercetin) and six phenolic acids (gallic acid, p-coumaric acid, ferulic acid, sinapic acid, vanillic acid, caffeic acid) were identified in the three samples (PP, Ch, & CCP). A total of nine polyphenol standards were applied (Figure 1). The wavelength used to detect (±)-catechin hydrate, (-)-epicatechin and sinapic acid was 280 nm, while 290 nm was applied with gallic acid, vanillic acid, caffeic acid, p-coumaric acid, trans-ferulic acid and quercetin hydrate. The chromatograms obtained for free polyphenol fractions (FPP) in PP, CCP and Ch are shown in Figure 2. Epicatechin, vanillic acid and sinapic acid were not detected in PP (Figure 2). However, Ch had significantly (P<0.05) greater amounts of epicatechin, vanillic acid, sinapic acid, gallic acid, catechin, caffeic acid and quercetin than CCP. Similarly, CCP showed the similar pattern with significantly (P<0.05) greater amounts of gallic acid, catechin, caffeic acid and quercetin than PP. p-coumaric acid concentrations in CCP (13.37 µg/g DW) and Ch (12.93 µg/g DWwere similar (P>0.05), and at the same time significantly (P<0.05) greater than that in PP (8.22 µg/g DW. Ferulic acid was the only polyphenol in PP with significantly (P<0.05) greater concentration compared with CCP and Ch (Table 2).
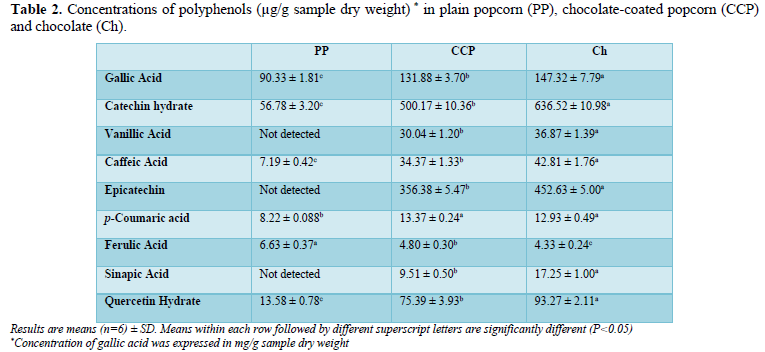
Gallic acid was the predominant polyphenol in all three samples (PP, CCP and Ch). More than 99% of FPP comprised of gallic acid. Consequently, gallic acid was expressed in mg/g sample DW, instead of µg/g sample DW, which was used with all other eight polyphenols. Following gallic acid, catechin was the second highest polyphenol in all three samples (Table 2). Gallic acid concentration in Ch (147.32 mg/g DW) was much higher than the concentration reported by Tokusoglu and Ünal (2002) in dark chocolate (0.57-0.72 mg/g FW).
Catechin concentration measured in chocolate (0.64 mg/g FW, equivalent to 636.52 µg/g DW) was within the values for baking chocolate (0.112-0.727 mg/g FW for chocolate (0.11-1.17 mg/g FW); and for sweetened chocolate (0.63-0.66 mg/g FW) [45]. On the other hand, catechin concentration was higher than in baking chocolate (0.24 mg/g FW), and lower than (1.848 mg/g FW) [33].
The detected amounts of vanillic acid in CCP and Ch in this current study (30.04 and 36.87 µg/g DW, respectively) were significantly greater (P<0.05) thanin chocolate milk (0.019 mg/g FW). However, both vanillic and sinapic acids were not detected in PP in this study.
Caffeic acid concentration in PP (7.19 µg/g DW) was much higher than those reported (0.013 µg/g DW). However, caffeic acid concentration was within the values reported in popcorn kernels 0.014 -9.83 µg/g DW. Caffeic acid concentration (0.0428 mg/g DW, equivalent to (7.19 µg/g DW) was much lower than the concentration reported in baking chocolate (0.32 mg/g FW). These discrepancies could be due to differences in the methods of analysis and extraction solvents, as different solvents will affect the recovery of polyphenols [46].
Epicatechin concentration in Ch (0.451 mg/g FW, equivalent to 0.453 mg/g DW) was within the range for baking chocolate (0.412-1.223 mg/g FW), but lower than the range (0.52 mg/g FW). Epicatechin is widely acknowledged as the main flavanol compound in chocolate. However, catechin concentration in Ch was higher than epicatechin [38,47] (Table 2).
P-coumaric acid concentration in PP (8.22 µg/g DW) was greater than the value reported in popcorn (0.005 µg/g DW), and smaller than the value in popcorn kernels (16.31 µg/g DW). Likewise, ferulic acid concentration in PP (6.63 µg/g DW) was greater than the amount (0.006 µg/g DW) in popcorn, and close to that in popcorn kernel (5.94 µg/g DW) [48].
Quercetin was detected in all tested samples (PP, CCP and Ch) with concentrations ranging from 93.27 µg/g DW in Ch to 13.58 µg/g DW in PP. The detected amount of quercerin in PP was much greater than the concentration in popcorn kernels (7.6 µg/g DW). It should mention also that neither detected quercetin in popcorn [49-52].
Due to limited standards availability at the time of this investigation, peaks 2 and 8 in PP and peaks 4 and 8 in CCP and Ch (Figure 2) were not identified.
CONCLUSION
The present study showed that covering popcorn with chocolate produced a snack with more polyphenol content and antioxidant capacity from chocolate and more fiber from popcorn. Consequently, the CCP can be considered a healthier snack than the indifidual PP and Ch [53-56].
Further investigation into other antioxidants such as carotenoids from popcorn, which is known to be rich in carotenods, is suggested. Additionally, bioaccessibility / bioavailability tests are recommended to determine the nutritional value of CCP compared with PP and Ch.
-
Bilman EM, van Trijp JCM, Renes RJ (2010) Consumer perceptions of satiety-related snack food decision making. Appetite 55(3): 639-347.
-
Watson WL, Kury A, Wellard L, Hughes C, Dunford E, et al. (2016) Variations in serving sizes of Australian snack foods and confectionery. Appetite 96: 32-37.
-
Fayet F, Mortensen A, Baghurst K (2012) Energy distribution patterns in Australia and its relationship to age, gender and body mass index among children and adults. Nutr Diet 69(2): 102-110.
-
Hooper L, Kay C, Abdelhamid A, Kroon PA, Cohn JS, et al. (2012) Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials. Am J Clin Nutr 95: 740-51.
-
Katz DL, Doughty K, Ali A (2011) Cocoa and chocolate in human health and disease. Antioxid Redox Signal 15: 2779-2811.
-
Nguyen V, Cooper L, Lowndes J, Melanson K, Angelopoulos TJ, et al. (2012) Popcorn is more satiating than potato chips in normal-weight adults. Nutr J 11: 71.
-
Min B, Gu L, McClung AM, Bergman CJ, Chen MH (2012) Free and bound total phenolic concentrations, antioxidant capacities, and profiles of proanthocyanidins and anthocyanins in whole grain rice (Oryza sativa L.) of different bran colours. Food Chem 133: 715-722.
-
Payne MJ, Hurst WJ, Miller KB, Rank C, Stuart DA (2010) Impact of fermentation, drying, roasting, and Dutch processing on epicatechin and catechin content of cacao beans and cocoa ingredients. J Agr Food Chem 58: 10518-10527.
-
Halvorsen BL, Carlsen MH, Phillips KM, Bohn SK, Holte K, et al. (2006) Content of redox-active compounds (i.e., antioxidants) in foods consumed in the United States. Am J Clin Nutr 84: 95-135.
-
Kaliora AC, Dedoussis GVZ, Schmidt H (2006) Dietary antioxidants in preventing atherogenesis. Atherosclerosis 187: 1-17.
-
Di Mattia CD, Sacchetti G, Mastrocola D, Serafini M (2017) From cocoa to chocolate: The impact of processing on in vitro antioxidant activity and the effects of chocolate on antioxidant markers in vivo. Front Immunol 8: 1207.
-
Özgüven MG, Berktaş I, Özçelik B (2016) Change in stability of procyanidins, antioxidant capacity and in-vitro bio accessibility during processing of cocoa powder from cocoa beans. LWT 72: 559-565.
-
Kofink M, Papagiannopoulos M, Galensa R (2007) Enantioseparation of catechin and epicatechin in plant food by chiral capillary electrophoresis. Eur Food Res Technol 225: 569-577.
-
Bernard R (2016) Snack Foods in the United States, Report, Agriculture and Agri-Food Canada (AAFC), Canada, viewed 1 March 2018.
-
Donkeun P, Allen KGD, Stermitz FR, Maga JA (2000) Chemical composition and physical characteristics of unpopped popcorn hybrids. J Food Compos Anal 13: 921-934.
-
Paraginski RT, de Souza NL, Alves GH, Ziegler V, de Oliveira M, et al. (2016) Sensory and nutritional evaluation of popcorn kernels with yellow, white and red pericarps expanded in different ways. J Cereal Sci 69: 383-91.
-
Sweley JC, Rose DJ, Jackson DS (2013) Quality traits and popping performance considerations for popcorn (Zea mays Everta). Food Rev Int 29: 157-177.
-
Karababa E (2006) Physical properties of popcorn kernels. J Food Eng 72: 100-107.
-
Quinn PV, Hong DC, Both JA (2005) Increasing the size of a piece of popcorn. Physica A 353: 637-48.
-
Food Standards Australia New Zealand (2014) Food Nutrient Database. AUSNUT – Australian Food, Supplement and Nutrient Database (AUSNUT) 2011-13 food nutrient database. Retrieved 16th April 2018, from FSANZ
-
Kljak K, Grbeša D (2015) Carotenoid content and antioxidant activity of hexane extracts from selected Croatian corn hybrids. Food Chem 167: 402-8.
-
Prasanthi PS, Naveena N, Rao MV, Bhaskarachary K (2017) Compositional variability of nutrients and photochemical in corn after processing. J Nutr Biochem 18: 567-579.
-
Sirisena S, Ng K, Jalousie S (2016) Antioxidant activities and inhibitory effects of free and bound polyphenols from date (Phoenix dactylifera L.) seeds on starchBilman digestive enzymes. Int J Food Stud 5: 212-223.
-
Garcia GB, Pardo GD, Arroqui C, Virseda P, Arroyo MRM, et al. (2015) Intra-laboratory validation of micro plate methods for total phenolic content and antioxidant activity on polyphenolic extracts, and comparison with conventional spectrophotometric methods. J Sci Food Agr 95: 204-209.
-
Tow WW, Premier R, Jing H, Ajlouni S (2011) Antioxidant and anti proliferation effects of extractable and no extractable polyphenols isolated from apple waste using different extraction methods. J Food Sci 76: T163-T172.
-
Gu L, House SE, Wu X, Ou B, Prior RL (2006) Procyanidin and catechin contents and antioxidant capacity of cocoa and chocolate products. J Agr Food Chem 54: 4057-4061.
-
Adam KK, Liu RH (2002) Antioxidant activity of grains. J Agr Food Chem 50:
-
Petronijević JL, Komes D, Gorjanović S, Cvitanović AB, Pezo L, et al. (2016) Content of total phenolics, flavan-3-ols and proanthocyanidins, oxidative stability and antioxidant capacity of chocolate during storage. Food Technol Biotech 54: 13-20.
-
Vertuani S, Scalambra E, Vittorio T, Bino A, Malisardi G, et al. (2014) Evaluation of antiradical activity of different cocoa and chocolate products: relation with lipid and protein composition. J Med Food 17: 512-516.
-
Miller KB, Stuart DA, Smith NL, Lee CY, McHale NL, et al. (2006) Antioxidant activity and polyphenol and procyanidin contents of selected commercially available cocoa-containing and chocolate products in the United States. J Agr Food Chem 54: 4062-4068.
-
Wu X, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, et al. (2004) Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J Agr Food Chem 52: 4026-4037.
-
Çelik EE, Gökmen V (2018) A study on interactions between the insoluble fractions of different coffee infusions and major cocoa free antioxidants and different coffee infusions and dark chocolate. Food chem 255: 8-14.
-
Meng CC, Jalil AMM, Ismail A (2009) Phenolic and theobromine contents of commercial dark, milk and white chocolates on the Malaysian market. Mol 14: 200-209.
-
Schwan RF, Wheals AE (2004) The microbiology of cocoa fermentation and its role in chocolate quality. Crit Rev Food Sci Nutr 44: 205-221.
-
Tabernero M, Serrano J, Calixto FS (2006) The antioxidant capacity of cocoa products: Contribution to the Spanish diet. Int J Food Sci Technol 4: 28-32.
-
Arranz S, Calixto FS (2010) Analysis of polyphenols in cereals may be improved performing acidic hydrolysis: A study in wheat flour and wheat bran and cereals of the diet. J Cereal Sci51: 313-318.
-
Hellström JK, Torronen AR, Mattila PH (2009) Proanthocyanidins in common food products of plant origin. J Agr Food Chem 57: 7899-7906.
-
Brcanovic JM, Pavlovic AN, Mitic SS, Stojanovic GS, Manojlovic DD, et al. (2013) Cyclic voltametric determination of antioxidant capacity of cocoa powder, dark chocolate and milk chocolate samples: correlation with spectrophotometric assays and individual phenolic compounds. Food Tech Biotechnol 51: 460.
-
Cervellati R, Greco E, Costa S, Guerra MC, Speroni E (2008) A comparison of antioxidant properties between artisan‐made and factory‐produced chocolate. Int J Food Sci Tech 43: 1866-1870.
-
Das AK, Sreerama YN, Singh V (2014) Diversity in photochemical composition and antioxidant capacity of dent, flint, and specialty corns. Cereal Chem 91: 639-645.
-
Mellor DD, Amund D, Georgousopoulou E, Naumovski N (2018) Sugar and cocoa: Sweet synergy or bitter antagonisms. Formulating cocoa and chocolate products for health: A narrative review. Int J Food Sci Tech 53: 33-42.
-
Wollgast J, Anklam E (2000) Review on polyphenols in Theobroma cacao: Changes in composition during the manufacture of chocolate and methodology for identification and quantification. Food Res Int 33: 423-447.
-
Xu JG, Hu QP, Wang XD, Luo JY, Liu Y, et al. (2010) Changes in the main nutrients, phytochemicals, and antioxidant activity in yellow corn grain during maturation. J Agr Food Chem 58: 5751-5756.
-
Alfieri M, Hidalgo A, Berardo N, Redaelli R (2014) Carotenoid composition and heterotic effect in selected Italian maize germplasm. J Cereal Sci 59:
-
Tokusoglu Ö, Ünal KM (2002) Optimized method for simultaneous determination of catechin, gallic acid, and methylxanthine compounds in chocolate using RP-HPLC. Eur Food Res Technol 215: 340-346.
-
Alothman M, Bhat R, Karim AA (2009) Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem 115.
-
Jolić SM, Redovniković IR, Marković K, Šipušić ĐI, Delonga K (2011) Changes of phenolic compounds and antioxidant capacity in cocoa beans processing. Int J Food Sci Technol 46: 1793-1800.
-
Žilić S, Serpen A, Akıllıoğlu G, Gökmen V, Vančetović J (2012) Phenolic compounds, carotenoids, anthocyanins, and antioxidant capacity of colored maize (Zea mays L) kernels. J Agr food chem 60.
-
Pandey R, Singh A, Maurya S, Singh U, Singh M (2013) Phenolic acids in different preparations of Maize (Zea Mays) and their role in human health. Int J Curr Microbiol App Sci 2: 84-92.
-
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, et al. (1999) Original Contributions: Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26: 1231-1237.
-
Risner CH, Kiser MJ (2008) High‐performance liquid chromatography procedure for the determination of flavour enhancers in consumer chocolate products and artificial flavours. J Sci Food Agr 88: 1423-1430.
-
Miller KB, Hurst WJ, Flannigan N, Ou B, Lee CY, et al. (2009) Survey of commercially available chocolate-and cocoa-containing products in the United States. 2. Comparison of flavan-3-ol content with nonfat cocoa solids, total polyphenols, and percent cacao. J Agr Food Chem 57: 9169-9180.
-
Hurst WJ, Krake SH, Bergmeier SC, Payne MJ, Miller KB, et al. (2011) Impact of fermentation, drying, roasting and Dutch processing on flavan-3-ol stereochemistry in cacao beans and cocoa ingredients. Chem Cent J 5: 53.
-
Cooper KA, Donovan JL, Waterhouse AL, Williamson G (2008) Cocoa and health: A decade of research. Br J Nutr 99: 1-11.
-
Australian Bureau of Statistics (ABS) (2014) Australian Health Survey: Nutrition First Results - Food and Nutrients. 2011-12.
-
Bayomy HM (2017) Sensory, Nutritional and Popping Qualities of Yellow and Purple Popcorn. J Food Dairy Sci 8(8): 361-367.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Genomic Medicine and Pharmacogenomics (ISSN:2474-4670)
- Journal of Veterinary and Marine Sciences (ISSN: 2689-7830)
- Journal of Womens Health and Safety Research (ISSN:2577-1388)
- Journal of Biochemistry and Molecular Medicine (ISSN:2641-6948)
- Journal of Agriculture and Forest Meteorology Research (ISSN:2642-0449)
- Journal of Genetics and Cell Biology (ISSN:2639-3360)
- Journal of Astronomy and Space Research




