Case Report
Sclerosing Cholangitis as Immune-Related Adverse Event Caused by Nivolumab and Ipilimumab Therapy
5378
Views & Citations4378
Likes & Shares
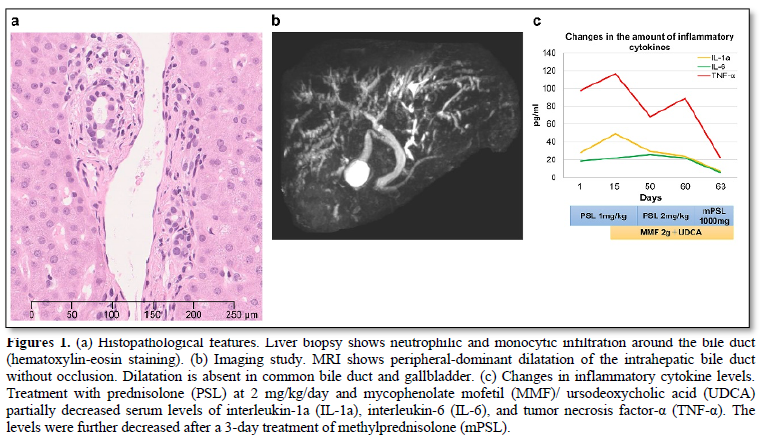
Several immune-related adverse events are known to cause immune checkpoint inhibitors (ICIs) are known to cause; however, few studies have described sclerosing cholangitis (SC) and its therapeutics. We report a patient with SC that was associated with nivolumab and ipilimumab combination therapy for advanced melanoma. A 62-year-old Japanese man had primary oral melanoma with bilateral cervical lymph node metastases. Combination therapy with nivolumab and ipilimumab was administered; however, six weeks after the fourth course, hepatic dysfunction developed. infiltration of neutrophils and monocytes around the bile ducts were observed on liver biopsy. Magnetic resonance imaging revealed peripheral-dominant dilatation of the intrahepatic bile ducts without occlusion. The patient was diagnosed with SC associated with nivolumab and ipilimumab combination therapy. Prednisolone (PSL) was initiated at 1 mg/kg/day. However, there was no improvement in hepatic dysfunction. The treatment egimen was then modified and mycophenolate mofetil (MMF) at 2 g/day and ursodeoxycholic acid (UDCA) was added followed by increased amount of PSL at 2 mg/kg/day. Moreover, intravenous methylprednisolone (1000 mg/day) was administrated for 3 days, and the bile duct enzymes ceased to increase. Inflammatory cytokine levels partially reduced after increasing PSL with MMF/UDCA. After methylprednisolone administration, the levels further reduced, which was parallel to the improvement in bile duct enzymes.
Keywords: Sclerosing Cholangitis, Immune-related adverse event, Nivolumab, Ipilimumab
INTRODUCTION
Immune checkpoint inhibitors (ICIs) cause several immune-related adverse events (irAEs); however, few studies have described sclerosing cholangitis (SC) and its therapeutics. We herein report a patient with SC that was associated with nivolumab and ipilimumab combination therapy for advanced melanoma.
CASE REPORT
A 62-years-old Japanese man had primary oral melanoma with bilateral cervical lymph node metastases. Nivolumab and ipilimumab were initiated after proton therapy. Diarrhea and colitis (grade 2) occurred after two courses of the treatment. These symptoms improved after administering 1 mg/kg/day of prednisolone (PSL), which was reduced to 10 mg/day over 2 months. No relapse of irAEs occurred; therefore, nivolumab and ipilimumab were resumed. Two weeks after the fourth course of treatment, nausea (grade 2) occurred. Subsequently, 1 month later, hepatic dysfunction developed with the following enzyme levels: aspartate aminotransferase, 81 U/L (normal range 13-30); alanine aminotransferase, 130 U/L (10–42); γ-glutamyl transpeptidase, 514 U/L (13-64); and alkaline phosphatase, 1163 U/L (106-322). Biopsied liver showed infiltration of neutrophils and monocytes around the bile ducts (Figure 1a). Magnetic resonance imaging (MRI) showed peripheral-dominant dilatation of the intrahepatic bile ducts without occlusion (Figure 1b). The patient was diagnosed with SC associated with nivolumab and ipilimumab combination therapy. PSL was initiated at 1 mg/kg/day. However, hepatic dysfunction did not sufficiently improve. Two weeks later, mycophenolate mofetil (MMF) at 2 g/day and ursodeoxycholic acid (UDCA) were added to the treatment regimen followed by increased amount of PSL at 2 mg/kg/day. Furthermore, intravenous methylprednisolone (1000 mg/day) was administrated for 3 days, and the increase in bile duct enzymes stopped. Inflammatory cytokine levels partially reduced after increasing PSL with MMF/UDCA. The levels further reduced after methylprednisolone administration (Figure 1c), which was parallel to the improvement in bile duct enzymes. Although the bile duct enzymes were not completely normalized, PSL was gradually tapered off to 0.4 mg/kg/day together with MMF discontinuation.
DISCUSSION
ICI-induced SC is a rare irAE and its diagnostic criteria and therapeutics have not been established [1,2]. Recently, clinical characteristics of ICI-induced SC have been reported [3]. In ICI-induced SC, men are affected twice as often as women. Abdominal pain and discomfort occasionally occur, and bile duct enzyme levels are remarkably increased compared to the liver enzymes. Intrahepatic bile duct dilatation without occlusion and infiltration of CD8+ T cells in and around the bile ducts are frequently observed [3,4]. Furthermore, ICI-induced SC does not respond well to corticosteroids. In our analysis, inflammatory cytokine levels increased in ICI-induced SC and the cytokine levels remarkably decreased after steroid pulse therapy, suggesting the significance of immunosuppression for the treatment. Corticosteroids are not sufficiently effective in ICI-induced SC; therefore, we need to clarify the inflammatory mechanism for an appropriate treatment. In addition, UDCA may be useful in reducing the onset of ICI-induced SC although it does not effectively improve SC after the onset [5].
-
Gelsomino F, Vitale G, D'Errico A, Bertuzzi C, Andreone P, et al. (2017) Nivolumab-induced cholangitic liver disease: A novel form of serious liver injury. Ann Oncol 28: 671-672.
-
Kawakami H, Tanizaki J, Tanaka K, Haratani K, Hayashi H, et al. (2017) Imaging and clinicopathological features of nivolumab-related cholangitis in patients with non-small cell lung cancer. Invest New Drugs 35: 529-536.
-
Takumi O, Yohei T, Taro Y, Hamamoto W, Sakamoto Y, et al. (2020) HLH-2004: Programmed cell death-1 inhibitor-related sclerosing cholangitis: A systematic review. World J Gastroenterol 26: 353-365.
-
Zen Y, Yeh MM (2019) Checkpoint inhibitor-induced liver injury: A novel form of liver disease emerging in the era of cancer immunotherapy. Semin Diagn Pathol 36: 434-440.
-
Zhu GQ, Shi KQ, Huang GQ, Wang LR, Lin YQ, et al. (2015) A network meta-analysis of the efficacy and side effects of UDCA-based therapies for primary sclerosing cholangitis. Oncotarget 6: 26757-26769.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Alcoholism Clinical Research
- Stem Cell Research and Therapeutics (ISSN:2474-4646)
- International Journal of Anaesthesia and Research (ISSN:2641-399X)
- International Journal of AIDS (ISSN: 2644-3023)
- Oncology Clinics and Research (ISSN: 2643-055X)
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)
- International Journal of Clinical Case Studies and Reports (ISSN:2641-5771)



