6171
Views & Citations5171
Likes & Shares
Type 2 diabetes mellitus (T2DM) is a major lifestyle disorder with a steep rise in the prevalence rates all over the world. India is epicentre of diabetes mellitus and most patients fail to achieve their glycaemic targets resulting in long term micro and macro vascular complications. Considering the complex pathophysiology, T2DM may not be managed efficiently through a glucocentric approach. Several new pharmacological options have been explored for better management of T2DM. Sodium-glucose co-transporter-2 inhibitors (SGLT2i) is one category, which acts by inhibiting SGLT2 co-transporter and prevents the reabsorption of urinary glucose thereby facilitating its excretion in urine. In India, SGLT2i are recommended in management of T2DM either as monotherapy or as combination therapy. Dapagliflozin, an SGLT2i, apart from glycaemic control is associated with non-glycaemic benefits such as cardioprotective, nephro-protective effects, delay progression of prediabetes to diabetes, and reduction in body weight, blood pressure. Safety and benefit/risk profile of dapagliflozin is predictable and favourable. This is a comprehensive review article of dapagliflozin summarizing the available evidence, not limited to glycaemia but extending to benefits beyond glycaemic control.
Keywords: Dapagliflozin, Oral hypoglycaemic agents, SGLT2 inhibitors, Type 2 diabetes mellitus
Abbreviations
ADA: American Diabetes Association; AEs: Adverse Events; ASCVD: Atherosclerotic cardiovascular disease; BP: Blood Pressure; CAD: Coronary Artery Disease; CANVAS: Canagliflozin Cardiovascular Assessment Study; CAP: Controlled Attenuation Parameter; CKD: Chronic Kidney Disease; CVD: Cardiovascular Disease; DAPA-HF: Dapagliflozin and Prevention of Adverse- Outcomes in Heart Failure; DBP: Diastolic blood pressure; DECLARE-TIMI58: Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58; DELIVER: Dapagliflozin Evaluation to Improve the LIVEs of Patients With Preserved Ejection Fraction Heart Failure; DEPICT 1: Dapagliflozin Evaluation in Patients With Inadequately Controlled Type 1 Diabetes; DKA: Diabetic Ketoacidosis; DKD: Diabetic Kidney Disease; DPP-4: Dipeptidyl Peptidase-4;EAT: Epicardial Adipose Tissue; EMPA-REG: Empagliflozin Cardiovascular Outcome Event Trial in T2DM Patients-Removing Excess Glucose; FDA: Food and drug administration; FPG: Fasting plasma glucose; ESRD: End-Stage Renal Disease; GLP-1: Glucagon-Like Peptide 1; GFR: Glomerular Filtration Rate; HFrEF: Heart Failure And Reduced Ejection Fraction; HFpEF: Heart Failure And Preserved Ejection Fraction; HR: Hazard Ration; Hb1Ac: Glycosylated haemoglobin; HDL: High-density lipoprotein; HF: Heart Failure; HHF: Hospitalization for Heart; IHD: Ischemic Heart Disease; KDIGO: Kidney Disease Improving Global Outcomes; KCCQ: Kansas City Cardiomyopathy Questionnaire; LDL: Low Density Lipoprotein; LSM: liver stiffness measurement; MACE: Major Adverse Cardiovascular Events; MI: Myocardial Infarction; NAFLD: Non-Alcoholic Fatty Liver Disease; OHA: Oral Hypoglycaemic Agents; OR: Odds ratio; OSAHS: Obstructive Sleep Apnea-Hypopnea Syndrome; PCT: Proximal Convoluted Tubule; PPG: Post-Prandial Plasma Glucose; SBP: Systolic Blood Pressure; SGLT2i: Sodium-Glucose Co-Transporter-2 Inhibitors; T2DM: Type 2 Diabetes Mellitus; UTIs: Urinary Tract Infections; VAT: Visceral Adipose Tissue.
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is a chronic condition with a worldwide prevalence of around 463 million in 2019 [1]. In India, around 77 million patients are suffering from diabetes [2]. India is epicentre of diabetes mellitus and has the world’s second-largest population of diabetes in 2017 which will double by 2045 [3]. Asian-Indian phenotype is characterized by obesity, hyperinsulinemia, insulin resistance, and atherogenic dyslipidaemia, which predispose Indians to T2DM and premature cardiovascular disease (CVD) [4]. Indian epidemiological evidence reported a substantial burden of vascular complications in diabetes. Neuropathy (24.6%) was the most common complication, followed by cardiovascular (23.6%), renal (21.1%), and ocular (16.6%) complications as perA1chieve study [6]. Heart failure (HF) risk was increased by 2.5 to 5-fold in T2DM. CVD was responsible for two-thirds of deaths in T2DM patients [5]. Several studies had exhibited around a 2-fold increased risk of myocardial infarction (MI), stroke, or ischemic heart disease (IHD) and a 2.35-fold higher risk of coronary artery disease (CAD) in patients with diabetes than non-diabetes [7,8]. In the 'Joint Asia Diabetes Evaluation' registry, people with diabetes from India ranked highest in the prevalence of chronic kidney disease (CKD) and second highest in the prevalence of albuminuria among 7 Asian countries [9].
The majority of diabetic patients on anti-diabetic drug therapy suffer from poor glycaemic control, dyslipidaemia, and diabetic vascular complications [10]. Combination therapy (54%) is most prescribed followed by monotherapy (46%) [11]. Most widely prescribed class of drug is sulfonylureas while metformin is a commonly prescribed individual drug among oral hypoglycaemic agents (OHA). Also, insulin and a fixed-dose combination of biguanide and sulfonylurea are prescribed and used frequently [10].
BACKGROUND
T2DM is a multi-organ disease arising from the combination of insulin resistance and a beta-cell secretory defect. Since the complications related to T2DM impact multiple organ systems and daily life significantly, reducing glycosylated haemoglobin (HbA1c) levels to the normal range is extremely crucial [12]. The uncontrolled glycaemia in T2DM aggravates the other pathophysiological processes in CVD or CKD which may lead to developing progressive vascular complication(s) (diabetic kidney disease (DKD) or diabetic cardiomyopathy) and may give rise to hypertension or dyslipidaemia [2, 13]. In India, around 70% of patients with diabetes in India have HbA1c ≥7; thus, fail to achieve the optimal glycaemic control as recommended by most guidelines [14].
The T2DM management strategy should achieve glycaemic control and decrease micro-and macrovascular outcomes. The approach of earlier T2DM management was glucocentric which contemplated hyperglycaemia as the primary target. Whereas several new classes of pharmacological agents focus on treating the under lying disease process and not only reacting to the blood glucose levels [12]. Since the advent of sulfonylureas in 1950, they have been a mainstay in T2DM treatment with metformin. However, recent evidence showed a lowering of glycaemic control after 6 months after the addition of sulfonylurea to metformin. The novel classes of drugs have shown glucose-lowering efficacy, higher safety, and long-term outcomes when added in patients having inadequate glycaemic control with metformin. Some are superior to sulfonylureas in lowering the risk of cardiovascular complications [15]. Sodium-glucose co-transporter-2 inhibitors (SGLT2i) is one such category, which is found effective in T2DM patients with insulin resistance and cardiovascular complications [16].
Gliflozins
Gliflozin drugs are the SGLT2i. SGLT2i are the recent class of OHA used in treating T2DM [17]. They act by inhibiting the SGLT2 in the proximal convoluted tubule (PCT); prevent the absorption of glucose and expedite its excretion in urine. This lowers blood glucose levels and improves glycaemic parameters [18].
Recently, EMPA-REG OUTCOME, DECLARE-TIMI and CANVAS trials demonstrated that SGLT2i reduce CVDs in T2DM patients. The evidence suggested that SGLT2i have a regulatory role in cellular stress, biochemical equilibrium, and inflammation in addition to lowering glycated haemoglobin and fasting blood glucose levels [17]. SGLT2i are related to improvements in a variety of cardiovascular risk factors such as a reduction in body weight, blood pressure (BP), waist circumference, and triglycerides, and an increase in high-density lipoprotein (HDL) cholesterol [19].
The pleiotropic effect in terms of cardiovascular benefits with SGLT2i is due to an improvement in ventricular loading caused by a reduction in cardiac load. The inhibition of glucose and sodium reabsorption in the PCT by SGLT2i causes an increase innatriuresis and glucosuria, followed by an increase in osmotic diuresis and reductions in preload. These effects result in reductions in BP and changes in vascular function. Apart from these most known mechanisms, complex cellular effects such as optimization of energy balance, oxidative stress down regulation, and pro-inflammatory signalling pathway modulation are associated with favourable outcomes observed in clinical trials [18, 20-21]. In addition, SGLT2i can change metabolism from glucose to oxidation of fatty acids and increase ketone plasma concentrations [22]. This fuel selection allows for more effective oxygen utilization and improved mitochondrial efficiency, which restores myocardial function in diabetics [23].
SGLT2i cause a substantial decrease in the risk of kidney disease progression relative to placebo in cardiovascular outcome trials in T2DM patients [18]. Increased natriuresis leads to higher sodium levels at the macula densa and triggerstubuloglomerular feedback which reduced renal blood flow and glomerular hyperfiltration [19,20]. Glial hyperfiltration is correlated to rapid function loss and glomerular filtration rate (GFR) decline in DKD and SGLT2i directly minimize it [20]. The position of SGLT2i in various guidelines is described in (Table 1).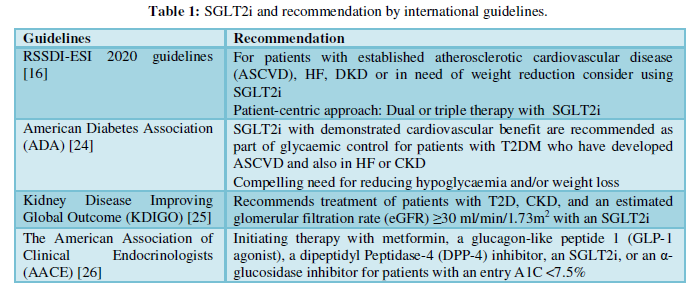
DAPAGLIFLOZIN PHARMACOLOGY
Dapagliflozin is a reversible and highly potent SGLT2i (inhibitory constant=0.55 nmol/L). It is >1400 times more selective to SGLT2 than SGLT1 [27]. Dapagliflozin improves glycaemic control and lowers bodyweight and BP in a wide variety of patients with T2DM, including those with elevated baseline HbA1c (9%) [28] and elderly (≥65 years) [29]. Dapagliflozin is administered orally with or without food and absorbed rapidly. The maximum peak plasma concentrations are reached within 2 hours (fasted state) [7]. The absolute oral bioavailability of dapagliflozin is 78% and mean plasma terminal elimination half-life of dapagliflozin is 12.9 hour at 10 mg dose. It is 91% protein-bound with a mean steady-state volume of distribution 118 l. It is metabolized by uridine diphosphate-glucuronosyltransferase 1A9 to its major inactive metabolite3-O-glucuronide in the liver and kidneys. The excretion of dapagliflozin and its metabolites occurs mainly via urine [27,30].
Dapagliflozin in T2DM management
Glycaemic efficacy trials Monotherapy
There have been several clinical trials reporting the efficacy of dapagliflozin when given as monotherapy (Table 2) [31-34]. Bailey et al. [31] conducted a 24-week randomized, double-blind, placebo-controlled, parallel group trial including 274 patients with baseline HbA1c (8.0%). They were randomized to receive placebo or dapagliflozin monotherapy 2.5 mg, 5 mg, or 10 mg once daily in the morning. After 24 weeks, low-dose metformin 500 mg/day was added to the placebo group therapy. The mean changes in HbA1c in the dapagliflozin 2.5, 5 and 10mg and placebo groups at 24 weeks were -0.58%, -0.77%, -0.89% and-0.23% (p values vs placebo: 0.0005, < 0.0001, and < 0.0001 respectively). These mean reductions in HbA1c and fasting plasma glucose (FPG) levels were maintained up to 102 weeks in the dapagliflozin 5mg (p=0.018 and 0.044) and 10mg (p=0.048 and 0.001 respectively) groups.
A pooled analysis of efficacy data from eight Phase IIb/III trials included 1453 Asian patients with T2DM, treated with placebo, dapagliflozin 5 mg, or dapagliflozin 10 mg over 24 weeks. It demonstrated that dapagliflozin was efficacious and well-tolerated in Asian patients with T2DM and showed similar results observed in diverse ethnicities. Greater reductions in HbA1c were seen with dapagliflozin 5 and 10 mg versus placebo at 24 weeks. At 24 weeks, placebo-corrected adjusted mean changes from baseline in HbA1c were -0.52%, and -0.58% for dapagliflozin 5 mg and 10 mg respectively [34] (Table2).
Add-on Therapy
Dapagliflozin has been studied as adjunctive therapy to several oral agents (Table 3) [35-40]. Yang et al. [35] reported that dapagliflozin+metformin had brought significant reductions in both Hb1Ac and FPG at week 24 compared to placebo (Table 2). A 24-week, randomized study conducted by Matthaei and colleagues [36] demonstrated superiority of dapagliflozin 10 mg/day with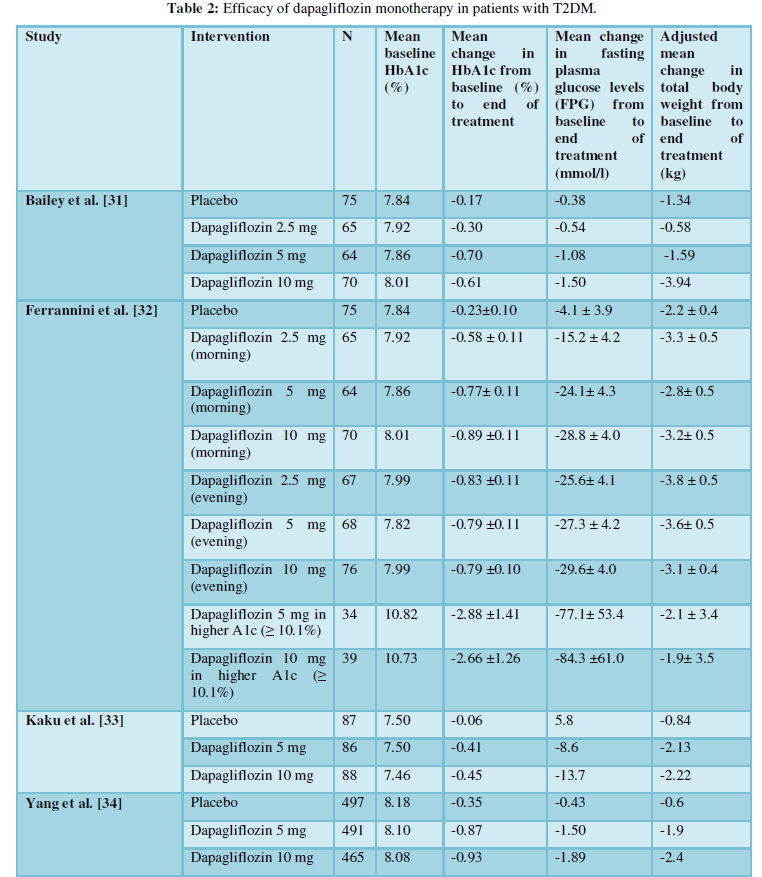
metformin and sulfonylurea in patients with inadequate glycaemic control on metformin and sulfonylurea. A significant reduction in HbA1c and FPG was reported at the end of 24 weeks from baseline (p=0.0001 for both groups). A large number of patients receiving dapagliflozin 10 mg/day were able to achieve a therapeutic glycaemic response (p=0.0001) than patients in the placebo group.
Jabbour et al [38] conducted a randomized, 24-week long study in 432 patients. The mean change in HbA1c from baseline was statistically significant in the dapagliflozin group ((10mg/day+sitagliptin) (100mg/day) ±metformin)) compared with placebo (sitagliptin (100mg/day) ±metformin) at week 24 (p=0.0001). The reduction in HbA1c in the dapagliflozin group was significant versus placebo when added to sitagliptin alone (p=0.0001) or to sitagliptin plus metformin (p=0.0001).
Rosenstock et al. [39] studied the effects of dapagliflozin when added with pioglitazone in a randomized, 24-week study (n=972). The discontinuation due to lack of glycaemic control took place mostly in placebo (34%) compared to dapagliflozin (11-18%). The reduction in HbA1c, FPG, and post-prandial plasma glucose (PPG) were significant in dapagliflozin 5and 10mg+pioglitazone compared with placebo+pioglitazone at week 24 and were maintained through week 48. The HbA1c decreased significantly in dapagliflozin+pioglitazone than the placebo+pioglitazone at week 24 (p=0.0007and p=0.0001 for dapagliflozin 5 and 10mg groups, respectively) and was maintained and found dose-dependent at 48 weeks. The fall in FPG was rapid and significant at week 24. At week 48, the mean change from baseline in PPG was greater with dapagliflozin+pioglitazone(-60.4 to -80.9 mg/dL [−3.35 to −4.49 mmol/L] than with placebo+pioglitazone (-25.4 mg/dL [1.41 mmol/L]).
In a placebo-controlled, double-blind study by wilding et al [40] randomly assigned 808 patients with T2DM inadequately controlled with insulin ³30 IU/day with or without up to two OHAs, to receive placebo or 2.5, 5 or 10 mg/day of dapagliflozin. Mean change in HbA1c from baseline at 104 weeks was -0.4% (p = 0.0002) and -0.4% (p = 0.0007) in the dapagliflozin 5/10 mg and 10 mg groups, respectively. Long-term reductions in HbA1c were evident at all dapagliflozin dosages. In patients receiving insulin and 1-2 OHAs, difference between the dapagliflozin 10-mg and placebo groups in HbA1c adjusted mean change from baseline was ((-0.4% (p=0.0050)) compared to patients on insulin alone ((0.3% (p=0.0563)). The mean reduction in placebo-adjusted mean changes in FPG from baseline at 104 weeks was −0.89 mmol/l (p=0.0031) and −0.31 mmol/l (p=0.3065) in the dapagliflozin 5/10-mg and 10-mg groups. There was an increase in insulin requirement in the placebo group (+18.3 IU/day), whereas it was stable throughout in the dapagliflozin groups. The difference in mean change in insulin daily-dose from baseline at 104 weeks from placebo were-16.8 IU (p<0.0001) and -19.2 IU (p<0.0001) in the dapagliflozin 5/10-mg and 10-mg groups, respectively.
Declare-TIMI 58 trial
The Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58 (DECLARE–TIMI 58) trial was conducted to assess the effects of dapagliflozin on CV and renal outcomes in patients aged ≥40 years with T2DM(HbA1c≥6.5 to <12%) and creatinine clearance ≥60 mL/min and established atherosclerotic cardiovascular disease (ASCVD)(40%) or multiple risk factors for ASCVD(60%). The primary efficacy outcomes were major adverse cardiovascular events (MACE) and the composite of cardiovascular death and hospitalization for heart (HHF). The two pre-specified secondary endpoints were renal composite outcome and death from any cause [41,42]. Dapagliflozin met the pre-specified criterion for non-inferiority with respect to MACE (P2 (HR=0.54; p<0·0001), end-stage renal disease (ESRD) (HR=0.31; p=0.013), renal death or ESRD (HR=0.41; p=0.012)) and reduced the progression of renal disease. The HbA1c values were lower in dapagliflozin than placebo recipients throughout the study (LS mean treatment difference=0.42%) [41,43] (Table 3).
Subgroup Analysis
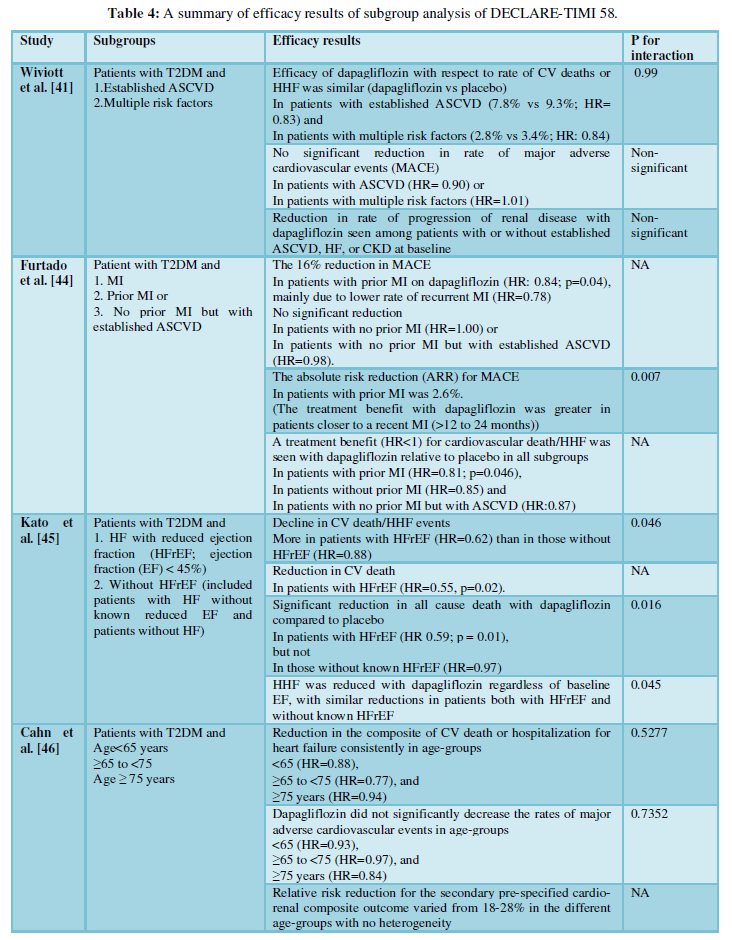
The different subgroup analyses of DECLARE-TIMI 58 are described in (Table 4).
Dapagliflozin - benefits beyond glycaemic control
Cardio protection
The Dapagliflozin and Prevention of Adverse- Outcomes in Heart Failure (DAPA-HF) trial showed that dapagliflozin in addition to standard of care reduced the risk of mortality and HF hospitalization. Trial enrolled 4,744 patients who had HFrEF (left ventricular EF≤40%). At baseline, 45% had T2DM.The combination improved symptoms in patients with HF and reduced ejection fraction (HFrEF). The patients were distributed according to the age groups i.e.<55, 55-64, 65-74, ≥75. There was significant reduction in the rate of cardiovascular death or HF hospitalization/urgent HF visit in the patients >55 years old (P<0.005; p for interaction in various age groups=0.75). The effect of dapagliflozin compared with placebo on cardiovascular death, HF hospitalization/urgent HF visit, all-cause death, and cardiovascular death/HF hospitalization recurrent events examined was consistent across all the categories of age (p-value for interaction=0.97). The patients treated with dapagliflozin showed greater improvement in Kansas City Cardiomyopathy Questionnaire (KCCQ-TSS) from baseline to 8 months; it was consistent across age categories (p-value for interaction=0.65). This result was important in elder people (≥75 years of age) as improvement or prevention of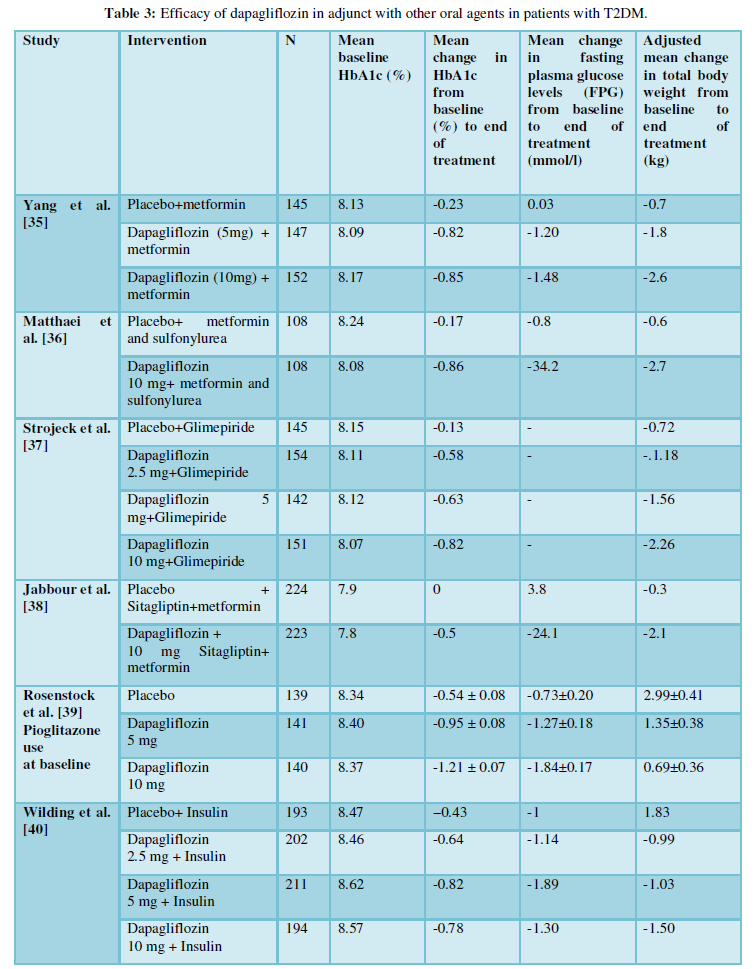
deterioration, in symptoms could be crucial as extending life. Also, these patients received slightly less conventional treatment, which further amplified the advantage of dapagliflozin in this study [47].
The cardio protective effects of dapagliflozin were further reinforced by CVD-REAL Nordic study (real-world data; n=40,908). Dapagliflozin caused significant reduction in the risk of MACE (non-fatal MI and stroke or CV mortality; HHF, and all-cause death (HR=0.79, 0.62, and 0.59 respectively) versus DPP-4 inhibitors after a mean follow-up of 0.95 years [48]. Berg et al [49] in patients with chronic HFrEF (n = 4744) evaluated the timing taken to the onset of clinical benefit with dapagliflozin. The reduction in the risk of cardiovascular death or worsening HF was rapid. Dapagliflozin reduced the relative risk of the primary outcome by 16% (HR=0.84), 27% (HR=0.73), and 36% (HR=0.64) in patients with a prior HF hospitalization never, >12 months ago, and 12 or fewer months ago, respectively (P=0.07 for trend). Accordingly, the absolute risk reduction was greater in patients with a more recent HF hospitalization at 2 years: 2.1%, 4.1%, and 9.9% respectively (P=0.05 for trend).
Nephro-protection
Reno-protective effects of SGLT2i have been studied and evident in patients with T2DM and CKD in the CREDENCE trial. DAPA-CKD trial enrolled 4304 CKD patients (estimated GFR: 25-75 ml/minute/1.73m2 of body-surface area; urinary albumin to creatinine ratio of 200-5000 mg/g) and 67.5% of them had T2DM at baseline. Primary renal composite outcome of a sustained decline in the estimated GFR (of at least 50%), end-stage kidney disease, or death from renal or cardiovascular causes were lower in the dapagliflozin group than the placebo group (HR= 0.61, P<0.001), when added to standard of care. Change in eGFR in dapagliflozin and placebo groups were -1.67±0.11 and -3.59±0.11 ml/minute/1.73 m2 respectively, for a between-group difference of 1.92 ml/minute/1.73m2/year. DAPA-CKD showed that dapagliflozin reduced the risk of worsening kidney function or death from cardiovascular or kidney disease in CKD patients with or without T2DM [50,51].
Other beneficial effects
Bodyweight
Several randomized trials in dapagliflozin as mono- and add-on therapy have shown a reduction in bodyweight as one of their outcomes (Table 1 and 2). Dapagliflozin is responsible for reducing body weight and is linked to a decrease in insulin requirement. The decline in weight is greater in patients with long diabetes history and higher baseline weight [16,52]. Analysis of 104-week data comparing dapagliflozin+insulin and placebo+insulin demonstrated that the total bodyweight increased progressively in the placebo group while it decreased in the dapagliflozin groups by 48 weeks. It was consistent over 104 weeks. The mean change in total bodyweight against placebo from baseline at 104 weeks were −2.86 kg (p<0.0001) and −3.33 kg (p<0.0001) in the dapagliflozin 5/10- mg and 10-mg groups, respectively [37]. Another analysis in patients receiving the add-on dapagliflozin 10 mg once daily showed a significant decrease in weight (p<0.0001), smaller waist circumference (p=0.0143), and less fat mass (p=0.0001) compared to add-on placebo at week 24. This study supported the findings that weight loss and reduction in fat mass with dapagliflozin were majorly due to caloric loss achieved from glucosuria [16,53]. A series of meta-analyses claimed that there was around 1-2 kg weight reduction with dapagliflozin and around 5 kg with metformin when compared against sulfonylurea [54-56].
Systolic blood pressure
Dapagliflozin caused reductions in 24-hour ambulatory BP monitoring, resulting in significantly lower systolic and diastolic BP (SBP and DBP) measurements [57]. A pooled analysis in asian populations showed reduction in seated SBP and DBP with dapagliflozin 5 and 10 mg versus placebo at 24 weeks. Placebo-corrected adjusted reductions were −2.5 and −3.1 mmHg in SBP, and −1.4 and −1.3 mmHg in DBP at 24 weeks with dapagliflozin 5 and 10 mg, respectively [34].
Obstructive sleep apnea
The prevalence of obstructive sleep apnea-hypopnea syndrome (OSAHS) in T2DM patients with obesity is 86%. The symptoms of OSAHS are apnea or reduced breathing during sleep. Tang et al [58] reported a significant reduction in apnea-hypopnea index, an increase in minimum oxygen saturation, and a decrease in Epworth somnolence scale score (p<0.05) in dapagliflozin and metformin group (n=18) but not in glimepiride and metformin group (n=18) over 24 weeks. Dapagliflozin and metformin aided in decreasing the patient’s weight, ameliorating the blood glucose, BP, and blood lipid levels. Dapagliflozin improved patient’s ventilation and daytime sleepiness, thus providing symptomatic relief inpatients [58, 59].
Non-alcoholic fatty liver disease
Non-alcoholic fatty liver disease (NAFLD) is the most prevalent chronic liver disease with an estimated prevalence of 25% worldwide; it affects more than 50% of patients with T2DM [60]. Arandomized trial of 57 patients with T2DM and NAFLD received dapagliflozin (5 mg/day) or standard treatment with no SGLT2i for 24 weeks. At week 24, visceral adipose tissue (VAT), controlled attenuation parameter (CAP), liver enzymes, and bodyweight reduced significantly in dapagliflozin group, but, not in the control group. The CAP was significantly decreased from baseline in dapagliflozin group than the standard group (92.4±18.7 vs. 102.2±13.2%, p=0.0429). The change in liver stiffness measurement (LSM) was non-significant in the dapagliflozin group (9.49 ±6.05 kPa to 8.01±5.78 kPa; p=0.0539). In 14 patients from this group, LSM decreased significantly (p=0.0158). Furthermore, the reduction in serum alanine aminotransferase and γ-glutamyltranspeptidase levels was found in dapagliflozin group, but not in the control group, accompanied with significant reduction in visceral fat mass in dapagliflozin group. This finding suggested an improvement in liver steatosis in patients with T2DM and NAFLD [61].
Epicardial adipose tissue
Epicardial adipose tissue (EAT) is a modifiable risk factor for T2DM related cardiovascular complications and an emerging therapeutic target. In 40 T2DM patients with CAD, EAT volume was compared prospectively between the dapagliflozin and conventional treatment groups during a 6-month period. At baseline, the EAT volumes in the dapagliflozin and conventional treatment groups were comparable with each other while it decreased significantly at follow-up; there duction was significantly greater in dapagliflozin group than the conventional treatment group (-16.4±8.3 vs. 4.7±8.8 cm3, p=0.01). In addition, the TNF-α level decreased significantly in the dapagliflozin group (p=0.04); this change was significantly greater in dapagliflozin group than the conventional treatment group (-0.5±0.7 vs. 0.03±0.3 pg/ml, p=0.03). The correlation between the changes in the EAT volume and body weight (correlation co-efficient (r) =0.71, p=0.01) and also between the EAT volume and TNF-α level (r=0.51, p=0.04) was significant. Thus, dapagliflozin managed to establish glycaemic control and improve the levels of systemic inflammation markers and reduce the risk of cardiovascular events [62].
Dapagliflozin in type 1 diabetes mellitus
Dapagliflozin Evaluation in Patients with Inadequately Controlled Type 1 Diabetes (DEPICT-1) was the first report of the long-term use of a selective SGLT2i as an adjunct to insulin for the treatment of type 1 diabetes. Patients were randomized into dapagliflozin 5 mg, 10 mg, and placebo groups. Dapagliflozin 5 mg and 10 mg reduced HbA1c (difference vs. placebo 20.33% and 20.36% respectively) and body weight (difference vs. placebo 22.95% and 24.54% respectively) significantly at 52 weeks. Although hypoglycaemia events were comparable across treatment groups, more patients in the dapagliflozin groups had events such as definite diabetic ketoacidosis (DKA) (4.0%, 3.4%, and 1.9% in dapagliflozin 5 mg, 10 mg, and placebo groups, respectively) [63].
Dapagliflozin in diabetes prevention
The subgroup of 2,605 patients with HFrEF of DAPA-HF trial with no prior history of diabetes, and an HbA1c<6.5% at baseline was randomized to dapagliflozin 10 mg daily or placebo. Dapagliflozin decreased the incidence of T2D by 32%; predominantly in individuals with prediabetes at baseline. In patients with prediabetes, mean HbA1c levels decreased by -0.08% with dapagliflozin and by -0.04% with placebo at 8 months. The additional benefits of SGLT2i include improved insulin sensitivity, reduction in hyperinsulinemia, and enhancement of pancreatic β-cell function. These mechanisms could be efficacious in lowering the risk of developing T2DM in prediabetes [64].
PRE-D trial randomized the patients with BMI≥25 kg/m2 (age=30-70 years) and prediabetesto dapagliflozin (10 mg once daily), metformin (1700 mg daily), interval-based exercise, or control. A small reduction in the mean amplitude of glycaemic excursions (MAGE) was found in the dapagliflozin group (17.1%, p=0.042) and exercise group (15.3%, p=0.067) compared to the control. There was a decline in MAGE by 17.2% (p=0.041) in the dapagliflozin group and 15.4% (p=0.065) in the exercise group compared to metformin after 13 weeks [65].
Dapagliflozin safety
Safety profile
A pooled analysis of safety data from 13 placebo-controlled trials reported a favourable and predictable tolerability profile of dapagliflozin over 24 weeks (Figure 1).The overall incidence of hypoglycaemia was 13.7% and 12.4% with dapagliflozin and placebo, predominant in patients receiving insulin as background therapy. Food and drug administration (FDA) had issued the warning in educating and increasing awareness abouteuglycaemic ketoacidosis among patients receiving SGLT2i as ketoacidosis episodes after use of SGLT2i were observed. Overall, adverse events (AEs) related to renal function were seen in3.2% and 1.8% of patients receiving dapagliflozin and placebo. Events of reduction in renal creatinine clearance or renal impairment were more with Dapagliflozin; however, most events were transient, of mild/moderate intensity. Genital infections were more frequent in dapagliflozin than placebo group. Fewer lower-limb amputations were observed in the patients in the dapagliflozin and control groups [66]. Small but similar proportion of patients in the dapagliflozin and all control groups reported fractures during study periods (1.2% in each group). A pooled data from 21 clinical studies comprising patients with a history of CVD reported that dapagliflozin wasn’t related to increased cardiovascular risk in patients with T2DM [67].
Risk vs benefit
Several meta-analyses on dapagliflozin have reported that dapagliflozin improves HbA1c by 0.50%, FPG by 1.1 mmol/L, reduces weight by 2 kg body mass index by 1.1%, and SBP/DBP by 4/2 mmHg. However, an increased risk of genitourinary (odds ratio (OR) 3.50) and urinary tract (OR=1.40) infections (UTIs) were observed [30, 55, 56, 68, 69]. Though certain AEs such as DKA and genital mycotic infections episodes were higher with SGLT2i, the absolute hike in these complications is lesser than the absolute risk reductions produced by SGLT2i [70]. The genital infections can be managed by standard treatments [71]. Overall, data from major outcome trials clearly suggested that SGLT2 inhibitors are not associated with an increased risk of UTIs [72]. Looking at the overall safety and efficacy profile, dapagliflozin has shown astonishing potential as an OHA and can also offer pleiotropic effects which not only resolve 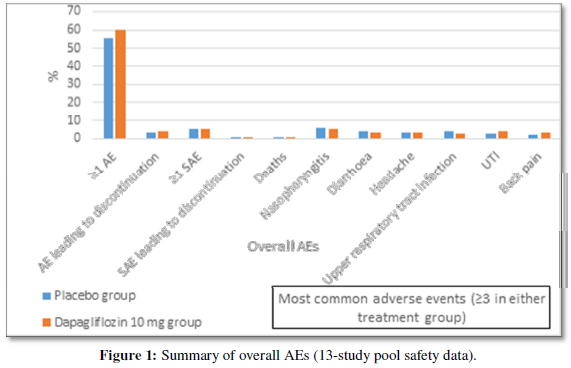
glycaemic disorders but also beneficial in treating other co-morbidities often present or developed with diabetes [73]. Dapagliflozin has shown favourable benefit/risk profile in all age categories [47].
When to avoid
There is no major evidence of SGLT2i exhibiting any clinically relevant drug-drug interactions with other OHAs. They can be concomitantly added or combined with metformin, sulfonylurea, pioglitazone, sitagliptin, and voglibose. However, ingestion of both loop diuretics and SGLT2i should be avoided in order to avoid hypotension and dehydration as they potentiate the risk of volume depletion [30]. The use of dapagliflozin isn’t recommended in breastfeeding women and the paediatric population as respective data are unavailable. Due to profound polyuria along with renal glycosuria observed with SGLT2i, they won’t be beneficial in pregnant women [74]. The co-administration of dapagliflozin with rifampicin led to a 22% decrease in dapagliflozin systemic exposure, but with no clinically meaningful effect on 24-hour urinary glucose excretion; thus, requiring no dose adjustment [75]. The use of dapagliflozin should be avoided in patients with higher DKA risk as it presents a risk of DKA episodes and volume depletion. There have been a number of case reports of euglycaemic ketoacidosis in the perioperative setting and hence, AACE has recommended not to ingestSGLT2i prior to surgery [76].
Place of dapagliflozin in clinical practice in the Indian context
A real-world evidence study in an Indian setting (FOREFRONT) [77] included 1978 Indian patients with T2DM to assess the efficacy and safety of dapagliflozin. A statistically significant reduction in HbA1c levels was observed from baseline to 3 and 6 months (P<0.001) irrespective of the HbA1c stratum (<8%, 8–10%, >10%). The effect was consistent in all Hb1Ac categories. Apart from the glycaemic effect, non-glycaemic effects such as weight loss (1.14 and 1.86 kg) and reduction in SBP (3.24 and 3.77 mmHg) were observed at the end of 3 and 6 months respectively. The RSSDI expert panel suggested that in Indian settings, SGLT2i are often recommended when metformin is not tolerated or contraindicated or when dual therapy is recommended (as a single agent is unable to achieve the glucose target), or when postprandial hypoglycaemia is a concern. The SGLT2i are recommended for benefits other than glycaemic control such as treating patients with comorbidities (established ASCVD, HF, DKD, or in need of weight reduction) [16]. Current ADA recommendations suggest SGLT2i and GLP-1 agonists as preferred add-on agents in addition to lifestyle interventions and metformin for diabetes patients with compelling indications for weight loss [24].
The use of dapagliflozin is not restricted solely to diabetes and its use has been approved for HFrEF and CKD in India [78-80]. Dapagliflozin is proven efficacious in T1DM as shown in DEPICT1 trial. A meta-analysis of DAPA-HF and EMPEROR trials observed the reduction in all-cause and cardiovascular death and the combined risk of cardiovascular death or worsening HF, as well as in the composite renal endpoint (HR=0.62) irrespective of the presence of diabetes or baseline eGFR [81]. The DIAMOND trial had shown SGLT2i’s effect on patients with CKD and without T2DM. An acute and reversible decline in measured GFR and a reduction in body weight were observed [82]. Looking at varied benefits of drugs of this class, cardiologists, nephrologists, endocrinologists, and primary care physicians should use these drugs while managing the cardio-renal disorders of their patients. As SGLT2i is newer class of drug, relying on multidisciplinary approach and gaining more experience can help lowering the barriers present in use of this therapy [83].
Lifelong treatment is needed in T2DM. Cost of treatment plays a major role while choosing the drugs in India as a large populace are devoid of medical insurance [16]. A Swedish study reported a lower hospital cost of US$321 per patient over 12 months associated with dapagliflozin, due to less cardiovascular risks, HF, and other T2D-related complications [84]. There have been ongoing trials which will fully evaluate therapeutic potential of SGLT2i in patients with or without T2DM. One such trial is DELIVER study; checking the impact of dapagliflozin on the rate of HHF and cardiovascular death in patients with heart failure preserved ejection fraction (HFpEF) and also, if those results will be able to complement DAPA-HF study [85]. Long-term clinical trials are being carried to find if SGLT2i can be efficient in major clinical kidney disorders in patients with CKD with and without diabetes [82].
CONCLUSION
In addition to glycaemic effects, presence of non-glycaemic effects (low risk of hypoglycaemia, concomitant weight loss, and the potential of lowering of BP) of dapagliflozin due to pleiotropic action make this molecule an attractive option in the treatment of T2DM. Apart from its use in diabetes with or without HF, CKD and ASCVD, the molecule has potential to change the management of CKD and HFrEF in patients without diabetes. The safety profile of dapagliflozin is favourable. Thus, dapagliflozin can be prescribed amongst broader range of T2DM patients with cardiac and renal disorders.
-
- International Diabetes Federation (2019) IDF Diabetes Atlas 9th Edn Available online at https://diabetesatlas.org/en/
- Das AK, Unnikrishnan AG, Saboo B, Mohan V, Vijay V (2018) Indian guidance on cardiovascular and renal comorbidity management in type-2 diabetes mellitus. Journal of The Association of Physicians of India 7.
- Oberoi S, Kansra P (2020) Economic menace of diabetes in India: a systematic review. Int J Diabetes Dev Ctries 1-12.
- Kalra S, Sahay RK, Schnell O, Sheu WH, Grzeszczak W, et al. (2013) Alpha-glucosidase inhibitor acarbose improves glycamic control and reduces body weight in type 2 diabetes: Findings on indian patients from the pooled data analysis. Indian J Endocrinol Metab 17: 307-9.
- Wang CC, Hess CN, Hiatt WR, Goldfine AB (2016) Clinical Update Cardiovascular Disease in Diabetes Mellitus: Atherosclerotic Cardiovascular Disease and Heart Failure in Type 2 Diabetes Mellitus - Mechanisms Management and Clinical Considerations Circulation 133: 2459-502.
- Mohan V, Shah S, Saboo B (2013) Current glycemic status and diabetes related complications among type 2 diabetes patients in India: data from the A1chieve study. J Assoc Physicians India 61: 12-5.
- Joshi SR, Vadivale M, Dalal JJ, Das AK (2011) The Screening Indias Twin Epidemic: Study design and methodology (SITE-1). Indian J Endocrinol Metab 15: 389-94.
- Mohan V, Venkatraman JV, Pradeepa R (2010) Epidemiology of cardiovascular disease in type 2 diabetes; The Indian Scenario. J Diabetes Sci Technol 4: 158-70.
- Luk AO, Li X, Zhang Y, Guo X, Jia W, et al. (2016) Quality of care in patients with diabetic kidney disease in Asia: The Joint Asia Diabetes Evaluation (JADE) Registry. Diabet Med 33: 1230-1239.
- Agarwal AA, Jadhav PR, Deshmukh YA (2014) Prescribing pattern and efficacy of anti-diabetic drugs in maintaining optimal glycemic levels in diabetic patients. J basic and clin pharm 5: 79.
- Sharma T, Sahai R, Bala S, Dhasmana DC, Kaeley N (2018) Prescribing pattern of oral anti-diabetic agents in type 2 diabetes mellitus patients in a tertiary care hospital. Int J Basic Clin Pharmacol 7: 956.
- Wyne KL (2004) Management of type 2 diabetes mellitus: is it time for a paradigm shift. Metab Syndr Relat Disord 2: 251-62.
- Petrie JR, Guzik TJ, Touyz RM (2018) Diabetes Hypertension and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can J Cardiol 34: 575-584.
- Unnikrishnan R, Anjana RM, Deepa M, Pradeepa R, Joshi SR, et al. (2014) Glycemic control among individuals with self-reported diabetes in India--the ICMR-INDIAB Study. Diabetes Technol Ther 16: 596-603.
- Genuth S (2015) Should Sulfonylureas Remain an Acceptable First-Line Add-on to Metformin Therapy in Patients With Type 2 Diabetes No Its Time to Move On. Diabetes Care 38: 170-175.
- Chawla R, Madhu SV, Makkar BM, Ghosh S, Saboo B, et al. (2020) RSSDI-ESI Clinical Practice Recommendations for the Management of Type 2 Diabetes Mellitus. Indian J Endocrinol Metab 24: 376.
- Tiwari S, Wadher S, Fardate S, Vikhar C (2019) Gliflozin a new class for type-II diabetes mellitus: an overview. Int J Pharm Sci & Res 10: 4070-77.
- Kalra S (2014) Sodium Glucose Co-Transporter-2 (SGLT2) Inhibitors: A Review of Their Basic and Clinical Pharmacology. Diabetes Ther 5: 355-366.
- Rios GV, Nadkarni GN (2020) SGLT2is: Emerging Roles in the Protection Against Cardiovascular and Kidney Disease Among Diabetic Patients. International journal of nephrology and renovascular disease 13: 281.
- Patel DK, Strong J (2019) The Pleiotropic Effects of Sodium Glucose Cotransporter-2 Inhibitors: Beyond the Glycemic Benefit. Diabetes Therapy 10:1771-1792.
- Lopaschuk GD, Verma S (2020) Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl Sci 5: 632-644.
- Ferrannini E, Baldi S, Frascerra S, Astiarraga B, Heise T, et al. (2016) Shift to Fatty Substrate Utilization in Response to Sodium-Glucose Cotransporter 2 Inhibition in Subjects Without Diabetes and Patients With Type 2 Diabetes. Diabetes 65: 1190-1195.
- Ferrannini E, Mark M, Mayoux E (2016) CV Protection in the EMPA-REG OUTCOME Trial: A Thrifty Substrate Hypothesis. Diabetes Care 39:1108-1114.
- American Diabetes Association (2020). Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes 2020. Diabetes 43: 98-110.
- Kidney Disease Improving Global Outcomes (KDIGO) (2020) Diabetes Work Group Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int 98: 1-115.
- Handelsman Y, Bloomgarden ZT, Grunberger G, Umpierrez G, Zimmerman RS, et al. (2015) American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for developing a diabetes mellitus comprehensive care plan executive summary. Endocrine Practice 21(4): 413-437.
- Dhillon S (2019) Dapagliflozin a review in type 2 diabetes. Drugs 79: 1135-1146.
- Skolnik N, Bonnes H, Yeh H, Katz A (2016) Dapaglifozin in the treatment of patients with type 2 diabetes presenting with high baseline A1C. Postgrad Med 128: 356-363.
- Fioretto P, Mansfeld TA, Ptaszynska A, Yavin Y, Johnsson E, et al. (2016) Long-term safety of dapaglifozin in older patients with type 2 diabetes mellitus: a pooled analysis of phase IIb/III studies. Drugs Aging 33: 511-22.
- Saeed M, Narendran P (2014) Dapagliflozin for the treatment of type 2 diabetes: A review of the literature. Drug Design Development and Therapy 8: 2493-2505.
- Bailey CJ, Morales Villegas EC, Woo V, Tang W, Ptaszynska A, et al. (2015) Efficacy and safety of dapagliflozin monotherapy in people with Type 2 diabetes: a randomized double-blind placebo-controlled 102-week trial. Diabet Med 32: 531-541.
- Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF (2010) Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized double-blind placebo-controlled phase 3 trial. Diabetes Care33: 2217-2224.
- Kaku K, Kiyosue A, Inoue S, Ueda N, Tokudome T, et al. (2014) Efficacy and safety of dapagliflozin monotherapy in Japanese patients with type 2 diabetes inadequately controlled by diet and exercise. Diabetes Obes Meta 16: 1102-1110.
- Yang W, Ji L, Zhou Z, Cain VA, Johnsson KM, et al. (2017) Efficacy and safety of dapagliflozin in Asian patients: A pooled analysis. J Diabetes 9: 787-799.
- Yang W, Han P, Min KW, Wang B, Mansfield T, et al. (2016) Efficacy and safety of dapagliflozin in Asian patients with type 2 diabetes after metformin failure: A randomized controlled trial. J Diabetes 8: 796-808.
- Matthaei S, Bowering K, Rohwedder K, Grohl A, Parikh S (2015) Study 05 Group Dapagliflozin improves glycemic control and reduces body weight as add-on therapy to metformin plus sulfonylurea: a 24-week randomized double-blind clinical trial. Diabetes Care 38: 365-72.
- Strojek K, Yoon KH, Hruba V, Elze M, Langkilde AM, et al. (2011) Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized 24-week double-blind placebo-controlled trial. Diabetes Obes Metab. 13: 928-938.
- Jabbour S, Hardy E, Sugg J, Parikh S (2014) Dapagliflozin Is Effective as Add-on Therapy to Sitagliptin With or Without Metformin: A 24-Wee Multicenter Randomized Double-Blind Placebo-Controlled Study. Diabetes Care 37: 740-750.
- Rosenstock J, Vico M, Wei L, Salsali A, List JF (2012) Effects of dapagliflozin an SGLT2i on HbA(1c) body weight and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care 35: 1473-1478.
- Wilding JP, Woo V, Rohwedder K, Sugg J, Parikh S, et al. (2014) Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years. Diabetes Obes Metab 16: 124-136.
- Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, et al. (2018) Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 380: 347-357.
- Raz I, Mosenzon O, Bonaca MP, Cahn A, Kato ET, et al. (2018) DECLARE-TIMI 58: Participants baseline characteristics. Diabetes Obes Metab 20: 1102-1110.
- Mosenzon O, Wiviott SD, Cahn A, Rozenberg A, Yanuv I, et al. (2019) Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: An analysis from the DECLARE–TIMI 58 randomised trial. Lancet Diabetes Endocrinol 7: 606-617.
- Furtado RHM, Bonaca MP, Raz I, Zelniker T, Mosenzon O, et al. (2019) Dapagliflozin and cardiovascular outcomes in patients with type 2 diabetes and prior myocardial infarction: a sub-analysis from DECLARE TIMI-58 Circulation 139: 2516-2527.
- Kato ET, Silverman MG, Mosenzon O, Zelniker TA, Cahn A, et al. (2019) Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation 139: 2528-2536.
- Cahn A, Mosenzon O, Wiviott SD, Rozenberg A, Yanuv I, et al. (2020)Efficacy and Safety of Dapagliflozin in the Elderly: Analysis From the DECLARE-TIMI 58 Study. Diabetes Care 4: 468-475.
- Martinez FA, Serenelli M, Nicolau JC, Petrie MC, Chiang CE, et al. (2020) Efficacy and Safety of Dapagliflozin in Heart Failure With Reduced Ejection Fraction According to Age: Insights From DAPA-HF. Circulation 141: 100-111.
- Persson F, Nystrom T, Jorgensen ME, Carstensen B, Gulseth HL, et al. (2018) Dapagliflozin is associated with lower risk of cardiovascular events and all-cause mortality in people with type 2 diabetes (CVD-REAL Nordic) when compared with dipeptidyl peptidase-4 inhibitor therapy: a multinational observational study. Diabetes Obes Metab 20: 344-351.
- Berg DD, Jhund PS, Docherty KF, Murphy SA, Verma S, et al. (2021) Time to Clinical Benefit of Dapagliflozin and Significance of Prior Heart Failure Hospitalization in Patients With Heart Failure With Reduced Ejection Fraction. JAMA Cardiol 207585.
- Heerspink H, Stefansson B, Correa-Rotter R, Chertow GM, Greene T, et al. (2020) Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med 383:1436-1446.
- Giorgino F, Vora J, Fenici P, Solini A (2020) Renoprotection with SGLT2 inhibitors in type 2 diabetes over a spectrum of cardiovascular and renal risk. Cardiovasc Diabetol 19: 196.
- Scheen A (2014) Pharmacokinetic and pharmacodynamic profile of empagliflozin a sodium glucose cotransporter 2 inhibitor. Clin Pharmacokinet 53(3): 213-25.
- Bolinder J, Ljunggren O, Kullberg J, Johansson L, Wilding J, et al. (2012) Effects of dapagliflozin on body weight, total fat mass and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J ClinEndocrinol Metab 97: 1020-1031.
- Zhang M, Zhang L, Wu B, Song H, An Z, et al. (2014) Dapagliflozin treatment for type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Metab Res Rev 30: 204-221.
- Goring S, Hawkins N, Wygant G, Roudaut M, Townsend R, et al. (2014) Dapagliflozin compared with other oral anti-diabetes treatments when added to metformin monotherapy: A systematic review and network meta-analysis. Diabetes Obes Metab 16: 433-442.
- Musso G, Gambino R, Cassader M, Pagano G (2012) A novel approach to control hyperglycemia in type 2 diabetes: Sodium glucose co-transport (SGLT) inhibitors: systematic review and meta-analysis of randomized trials. Ann Med 44: 375-393.
- Ott C, Jumar A, Striepe K, Friedrich S, Karg MV, et al. (2017) A randomised study of the impact of the SGLT2 inhibitor dapagliflozin on microvascular and macrovascular circulation. Cardiovasc Diabetol 16: 27.
- Tang Y, Sun Q, Bai X-Y,Zhou Y-F, Zhou QL, et al. (2019) Effect of dapagliflozin on obstructive sleep apnea in patients with type 2 diabetes: A preliminary study. Nutrition & Diabetes 9: 32.
- Sun Q, Tang Y, Zhand M (2019) 1195-P: Effect of Dapagliflozin on Obstructive Sleep Apnea in Patients with Type 2 Diabetes: A Randomized Controlled Trial. Diabetes 68.
- GameilMA, Abdelgawad MS, Bahgat MH, Elsebaie AH, Marzouk RE (2020) Influence of sodium glucose co-transporter 2 inhibitors on fatty liver index parameters in type 2 diabetes mellitus. Egypt J Intern Med 32: 12.
- Shimizu M, Suzuki K, Kato K, Jojima T, Iijima T, et al. (2019) Evaluation of the effects of dapagliflozin a sodium-glucose co-transporter-2 inhibitor on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes Metab 21: 285-292.
- Sato T, Aizawa Y, Yuasa S, Kishi S, Fuse K, et al. (2018) The effect of dapagliflozin treatment on epicardial adipose tissue volume. Cardiovasc Diabetol 17: 6.
- Dandona P, Mathieu C, Phillip M, Hansen L, Tschöpe D, et al. (2018) Efficacy and Safety of Dapagliflozin in Patients with Inadequately Controlled Type 1 Diabetes: The DEPICT-1 52-Week Study. Diabetes Care 41: 2552-2559.
- Inzucchi SE, Docherty KF, Kober L, Kosiborod MN, Martinez FA, et al. (2021) Dapagliflozin and the Incidence of Type 2 Diabetes in Patients with Heart Failure and Reduced Ejection Fraction: An Exploratory Analysis from DAPA-HF. Diabetes Care 44: 586-594.
- Færch K, Blond MB, Bruhn L, Amadid H, Vistisen D, et al. (2021) The effects of dapagliflozin, metformin or exercise on glycaemic variability in overweight or obese individuals with prediabetes (the PRE-D Trial): a multi-arm randomised controlled trial. Diabetologia 64: 42-55.
- Jabbour S, Seufert J, Scheen A, Bailey CJ, Karup C, et al. (2018) Dapagliflozin in patients with type 2 diabetes mellitus: A pooled analysis of safety data from phase IIb/III clinical trials. Diabetes Obes Metab 20: 620-628.
- Ptaszynska A, Johnsson K M, Parikh S J, De Bruin TW, Apanovitch AM, et al. (2014) Safety Profile of Dapagliflozin for Type 2 Diabetes: Pooled Analysis of Clinical Studies for Overall Safety and Rare Events. Drug Safety37: 815-829.
- Sun YN, Zhou Y, Chen X, Che WS, Leung SW (2014) The efficacy of dapagliflozin combined with hypoglycaemic drugs in treating type 2 diabetes mellitus: Meta-analysis of randomised controlled trials. BMJ Open 4: 004619.
- Vasilakou D, Karagiannis T, Athanasiadou E, Mainou M, Liakos A, et al. (2013) Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 159: 262-274.
- Johansen ME, Argyropoulos C (2020) The cardiovascular outcomes heart failure and kidney disease trials tell that the time to use Sodium Glucose Cotransporter 2 inhibitors is now. Clin Cardiol 43: 1376-1387.
- Unnikrishnan AG, Kalra S, Purandare V, Vasnawala H (2018) Genital Infections with Sodium Glucose Cotransporter-2 Inhibitors: Occurrence and Management in Patients with Type 2 Diabetes Mellitus. Indian J Endocrinol Metab 22: 837-842.
- Sarafidis PA, Ortiz A (2019) The risk for urinary tract infections with sodium-glucose cotransporter 2 inhibitors: No longer a cause of concern. Clin Kidney J 13: 24-26.
- Pittampalli S, Upadyayula S, Mekala HM, Lippmann S (2018) Risks vs Benefits for SGLT2i Medications. Fed Pract 35: 45-48.
- Toka HR, Yang J, Zera CA, Duffield JS, Pollak MR, et al. (2013) Pregnancy-associated polyuria in familial renal glycosuria. Am J Kidney Dis 62: 1160-1164.
- Kasichayanula S, Liu X, LaCreta F, Griffen SC, BoultonDWl (2014) Clinical Pharmacokinetics and Pharmacodynamics of Dapagliflozin a Selective Inhibitor of Sodium-Glucose Co-transporter Type 2. Clin Pharmacokinet 53: 17-27.
- Handelsman Y, Henry RR, Bloomgarden ZT, Jack SD, DeFronzo RA, et al. (2016) American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the association of sglt-2 inhibitors and diabetic ketoacidosis. Endocr Pract 22: 753-762.
- Viswanathan V, Singh K P (2019) Use of Dapagliflozin in the Management of Type 2 Diabetes Mellitus: A Real-World Evidence Study in Indian Patients (FOREFRONT). Diabetes Technology & Therapeutics 21: 415-422.
- Jhund P, Solomon S, Docherty K, Heerspink H, Anand I, et al. (2020) Efficacy of Dapagliflozin on Renal Function and Outcomes in Patients With Heart Failure With Reduced Ejection. Fraction Circulation 143: 298-309.
- AstraZeneca’s Dapagliflozin receives Marketing Authorisation for Chronic Kidney Disease in India (2021). Available online at https://www.astrazeneca.in/media/press-releases/2020/astrazenecas-dapagliflozin-receives-marketing-authorisation-for-chronic-kidney-disease-in-india
- AstraZeneca’s Dapagliflozin (Forxiga) approved in India for treatment of patients with Heart Failure with reduced ejection fraction (2020). Available online at https://www.astrazeneca.in/content/az-in/media/press-releases/2020/astrazenecas-dapagliflozin-forxiga-approved-in-india-for-treatment-of-patients-with-heart-failure-with-reduced-ejection-fraction.html.
- Fernandez-Fernandez B, Sarafidis P, Kanbay M, Navarro-Gonzalez JF, Soler MJ, et al. (2020) SGLT2 inhibitors for non-diabetic kidney disease: drugs to treat CKD that also improve glycaemia. Clin Kidney J 13: 728-733.
- Cherney DZI, Dekkers CCJ, Barbour SJ, Cattran D, Abdul Gafor AH, et al. (2020) DIAMOND investigators. Effects of the SGLT2 inhibitor dapagliflozin on proteinuria in non-diabetic patients with chronic kidney disease (DIAMOND): A randomised double-blind crossover trial. Lancet Diabetes Endocrinol 8: 582-593.
- Melillo G (2020) Panelists Argue for SGLT2 Inhibitors Uptake Among Nephrologists. AJMC Available online at https://www.ajmc.com/view/panelists-argue-for-sglt-2-inhibitors-uptake-among-nephrologists.
- NorhammarA, Bodegard J, Nyström T, Thuresson M, Rikner K, et al. (2019) Dapagliflozin vs non-SGLT-2i treatment is associated with lower healthcare costs in type 2 diabetes patients similar to participants in the DECLARE-TIMI 58 trial: a nationwide observational study. Diabetes Obes Metab 21: 2651-2659.
- Williams DM, Evans M (2020) Dapagliflozin for Heart Failure with Preserved Ejection Fraction: Will the DELIVER Study Deliver. Diabetes Ther 11: 2207-2219.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Otolaryngology and Neurotology Research(ISSN:2641-6956)
- International Journal of Medical and Clinical Imaging (ISSN:2573-1084)
- Advance Research on Endocrinology and Metabolism (ISSN: 2689-8209)
- Chemotherapy Research Journal (ISSN:2642-0236)
- Journal of Allergy Research (ISSN:2642-326X)
- Journal of Neurosurgery Imaging and Techniques (ISSN:2473-1943)
- Journal of Rheumatology Research (ISSN:2641-6999)



