Research Article
Ketone Bodies in the Fetus and Newborn During Gestational Diabetes and Normal Delivery
3874
Views & Citations2874
Likes & Shares
Background: Authors successfully treated gestational diabetes by a very low carbohydrate diet without insulin and other drugs. Increased ketone bodies seemed to play an essential role in energy metabolism, and the fetus and newborn also showed hyperketosis. It is necessary to clarify how much ketone bodies were present in the placenta and umbilical cord in the fetus and newborn and the pregnant mother with or without gestational diabetes.
Subjects and Methods: All cases were patients of Muneta OB/GYN Clinic in Chiba, where about 700 deliveries were done every year, 90% normal and 10% gestational diabetic. Blood of 313 mothers and babies at health check-up postpartum, 192 samples of placenta and cord blood at the delivery, and 122 cases were obtained at the time of miscarriage. Abbott's kit measured βHB, and 101 samples obtained at the post partem health check-up were biochemically analyzed for both βHB and glucose. The IBM-SPSS did the statistical analysis.
Results: βHB in Mothers' and newborns' blood at four days postpartum was 0.062 and 0.244 mmole/L (median), respectively, and glucose was 4.55±0.81 mmole/L. βHB was high throughout the pregnancy; In the placenta, βHB in the first-, second-and-third trimester was 1.95±0.9 mmole/L, 2.82±0.49 mmole/L, 1.87±0,65 mM/L, respectively. In the cord blood, it was 2.3±1.13 mmole/L, 1.36±0.76 mmole/L, and 0.69±0.6 mmole/L, respectively. Placental βHB at the delivery was 1.99±0.78 mmole/L, and that of the umbilical cord was 0.75±0.36 mmole/L. In the first trimester miscarriage, βHB in spontaneous abortion was 1.84±0.85 mmole/L, while it was 2.09±0.94 mmole/L in artificial abortion. Aborted cases in the second trimester showed 1.96±0.38 mmole/L βHB and 3.74±0.75 mmole/L glucose in the cerebrospinal fluid.
Discussion: Our data showed βHB and glucose concentration in the human fetus and newborn under the normal physiological condition. βHB was present in the placenta and umbilical cord blood throughout fetal life and after birth. Different concentrations between the placenta and umbilical cord blood suggested the fetus's uptake for energy and intrauterine growth. High βHB in the cerebrospinal fluid suggested the effects on neuronal development.
Keywords: 3-hydroxybutyric acid, Ketone bodies fetus, Placenta, Umbilical cord, Cerebrospinal fluid, Miscarriage, Gestational diabetes
Abbreviations: βHB: 3-hydroxybutyric acid
INTRODUCTION
In recent years, the prevalence of gestational diabetes has been increasing due to the obesity epidemic, late marriage, and late childbirth. When pregnant women with diabetes mellitus and with gestational diabetes are combined, about 10-15% of all pregnant women are associated with diabetes during pregnancy [1,2]. The incidence of preeclampsia, macrosomia, and shoulder dystocia should increase without proper treatment. When diet and lifestyle intervention do not result in adequate glucose control, treatment with insulin begins. We succeeded in the treatment of gestational diabetes by the super-low carbohydrate diet [3,4]. On that occasion, hyperketosis occurred in mothers. A low level of ketones, especially beta-hydroxybutyric acid (βHB), is present, so diabetic ketoacidosis has been worried. Mother's body fat changes are of particular importance. In early pregnancy, the mother's body accumulates adipose tissue supply for the fetus. A pregnant woman's appetite is strongly stimulated by increased placental lactogen, progesterone, and prolactin production. Acceleration of insulin production is also supportive. Hyperinsulinemia stimulates lipogenesis and decreases lipolysis, which results in the accumulation of fat tissue. In the second half of pregnancy, catabolic processes are observed by increased placental hormones and cytokine concentration [5,6]. The availability of free fatty acids increases for the maternal energy source by lipolysis according to the fetus's increased glucose requirement. These mechanisms are responsible for increased ketogenesis during pregnancy and are three times higher at night among pregnant women than among nonpregnant women [7,8]. On that occasion, hyperketosis occurred, mainly βHB increased. βHB becomes an energy source instead of glucose [9,10]. Fasting increased the βHB level by the decreased glucose level [11,12]. Our super-low carbohydrate diet comprises MEC (meat, egg, cheese) and coconut oil, and the bodyweight of gestational diabetic patients decreased without a hungry feeling [13]. Postprandial hyperglycemia does not occur, and insulin treatment was not necessary. The complication of gestational diabetes does not appear in obese pregnant women, and all our cases achieved a smooth standard delivery [14]. To know the effects of a mother's ketosis on the fetus, we studied the βHB concentration of the placenta and umbilical cord blood throughout fetal life and after delivery. The Association of British Clinical Diabetologists defines βHB blood concentration as the best way to monitor diabetic ketoacidosis. However, the American and the Polish Diabetes Association are not in favor of any of these methods [15,16].
SUBJECTS AND METHODS
All cases were patients of Muneta OB/GYN Clinic in Chiba, where about 700 deliveries were done every year, about 90% normal and 10% gestational diabetic. Clinical summary and anthropometric data of mothers, course of pregnancy, and delivery records were made from the clinical chart. All mothers who participated in this study gave written informed consent for measuring ketone and glucose of their baby and fetus in miscarriage. Three hundred thirteen cases provided both mothers’ and newborns’ blood to compare βHB concentration. We obtained one hundred-one samples at the post partem health check-up (blood only). Eighty-four samples of placenta and cord blood were obtained for βHB measurement at the delivery. Besides, 174 fetuses provided placenta and cord blood. We also received cerebrospinal fluid from 11 fetuses at spontaneous and artificial miscarriage. We summarized all data in the Excel database and the statistical analysis done by IBM-SPSS ver. 25 Measurement of βHB and glucose We applied Abbott's Precision Exceed Kit to measure βHB and glucose (No. 44562003, registration 22300AMX00567000; Abbott Japan, Kashiwa, Chiba, Japan) [17]. The usual range of βHB in a healthy person's blood is less than 0.085 mmole/L, and its measurement range is from 0.03 to 8.0 mmole/L. This kit's detailed information can be obtained in the HP of Abbott Co., Inc. [18]. Abbott's Precision Kit measured glucose at the same time. Fresh placental tissue fluid was sampled by pressure for measurement. The accuracy and reliability were confirmed by the comparison between Abbott's kit and biochemical analysis. The regression equation was Y=1.0372X+354.23 (R2=0.8668).
Statistical analyses
All data were summarized in the Excel database, and the statistical analysis was done by SPSS ver. 25 [19]. The average, standard deviation, the median of each variable was calculated. Correlation analysis between the placenta and umbilical cord βHB was done. Boxplot graphs were used to show a non-parametric distribution. Data were shown as the mean ± standard deviation or median when the distribution was not normal. The statistical significance level was set at p<0.05.
Ethical considerations
This study was conducted in compliance with ethical principles based on the Declaration of Helsinki, the Ethical Guidelines for Research in the medical field for Human beings, and the Good Clinical Practice (GCP) conduction. “Ethical Guidelines for Epidemiology Research” proposed by the Ministry of Education, Culture, Sports, Science, and Technology, and the Ministry of Health, Labor, and Welfare were also applied. The Ethical Committee approved the research plan in the Life science Promoting Association (#2016-3) and the Ethical Committee for the Clinical Research in the Muneta Maternal Clinic. This investigation has been registered by the National University Hospital Council of Japan, which is # R000046730.
RESULTS
βHB after the delivery was 0.062 mmole/L (median) in mothers’, and 0.156 mmole/L (median) in newborn. (Table 1). Correlation between mother and child βHB was significantly high, but paired analysis showed no significant correlation (p=0.135). Mothers’ total ketone bodies were positively correlated with mother’s βHB and baby's total ketones but negatively correlated with baby's βHB. βHB occupied 86.5±6.9% of total ketones in babies, but it was 67.3±12.2% in mothers.
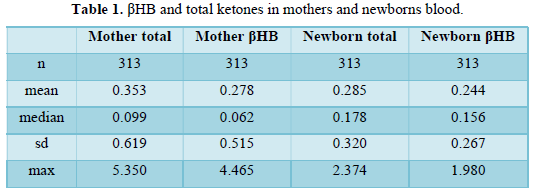

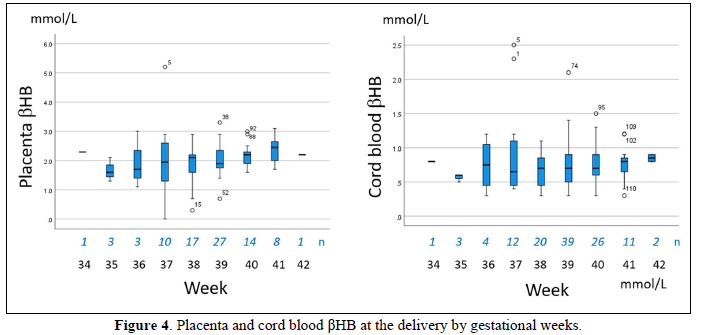
Four days after the delivery, the βHB of newborn blood was 0.24±0.12 mmole/L, 0.10-0.80 mmol/L in range, and glucose was 4.55±0.81 mmole/L (Figures 1 & 2). For the newborn at 30 days, the βHB value was 0.37 mmol/L on average, 0.40 mmol/L in the median, and 0.20-0.70 mmol/L in the range. The bodyweight of the newborn was 3085±440 g. Placental βHB at the delivery was 1.99±0.78 mmole/L, and umbilical cord blood was 0.75±0.36 mmole/L (Figure 3). The concentration of βHB was about one-third of placental βHB.
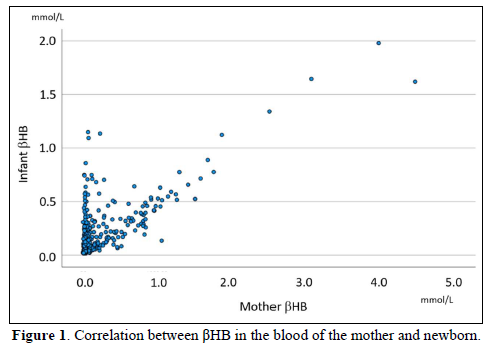
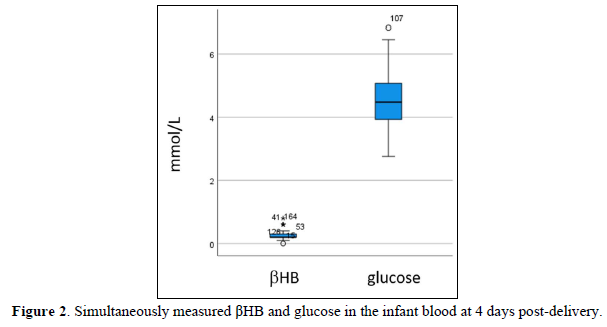
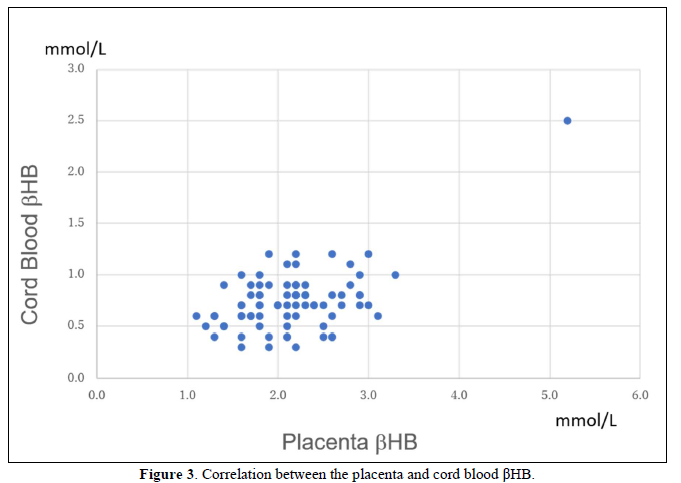



Placental and cord blood βHB were relatively stable in the third trimester, but it seemed to increase at the late delivery.
In the first and second trimester miscarriage, βHB in spontaneous abortion was 1.84±0.85 mmole/L, while it was 2.09±0.94 mmole/L and 3.10 mmole/L in artificial abortion.
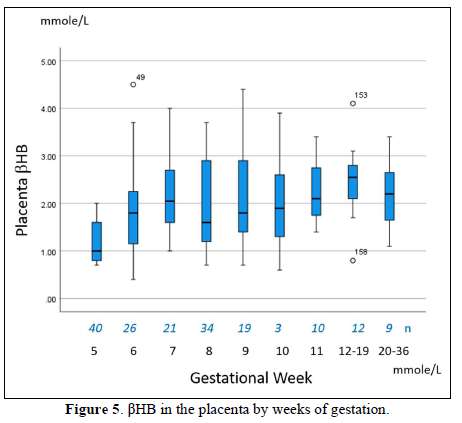
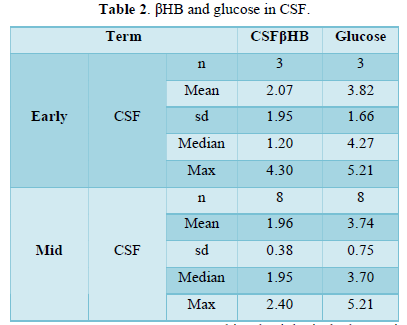
The placenta contained high βHB ranging from 1-3 mmole/L (Figures 4 & 5). It increased from 5 to 7 weeks, then stable during 8-11 weeks, and highest in the second trimester. Before delivery, it became around 2 mmole/L. In spontaneous miscarriage, βHB of the placenta and cord blood was 1.84±0.85 mmole/L and 1.5 mmole/L, respectively, while it was 2.09±0.94 mmole/L 3. 10 mmole/L by artificial abortion. Spontaneous abortion tended to show a low value of βHB (p=0.08). Aborted cases in the second trimester provided the cerebrospinal fluid, in which βHB was 1.96±0.38 mmole/L and glucose 3.74±0.75 mmole/L (Table 2).



DISCUSSION
Progesterone, prolactin, and placental lactogen produce increased appetite in pregnant women to become obese [20,21]. In addition, hyperinsulinemia would cause increased lipogenesis and decreased lipolysis, leading to the accumulation of fat tissues. In the first trimester of pregnancy, a pregnant woman often encounters morning sickness [22]., about 60% of women have complained of various degrees of discomfort, nausea, headache, and appetite loss. Approximately 10% of them have severe symptoms of vomiting, weakness, and fatigue. βHB is often elevated in these cases, such as 3.0-4.0 mmol/L in blood, where it was believed to be due to fasting and dehydration. This physiological change is considered to be a risk of gestational diabetes. The prevalence is increasing in parallel with the obesity epidemic. We succeeded in the treatment of gestational diabetes by a super-low carbohydrate diet [23]. At that time, pregnant women caused hyperketonemia, but no clinical symptoms happened. Instead, according to weight loss, the patient became vivid and completed an expected delivery. Muneta found pregnant women were easy to become hyperketonemia. At delivery, he measured βHB and glucose in the newborn blood and found βHB remained high complementing the glucose. βHB and glucose in the infant blood at the 4th-day health check-up were 0.24±0.12 mmole/L and 4.54±0.80, respectively. Then, the βHB of the placenta and umbilical cord blood was measured from the miscarriage fetus. In the fetal life, the placenta's median βHB was 1.8, 2,6, and 1.7 in the first, second, third trimester, respectively, and those of cord blood were 2.3, 1.35, and 0.60 mmole/L, respectively. Continuous βHB production in the placenta and decreasing concentration in the cord blood may be reflected by the increase of the fetus's requirement. Glucose has been considered to be the primary substrate for the placenta and fetal tissues. Glucose contributes to placental and fetal glycogen production and can exchange with other carbon-containing compounds at the three-carbon level of glycolysis. Directly measured lamb’s placental oxygen consumption is about 85%, and the indirect lamb's fetal oxygen consumption by glucose plus lactate is about 65%. Control of glucose supply to the placenta and fetus is far more complex than a simple direct relation with maternal glucose concentration. In fasting, when the blood glucose level decreased below 4.0 mmole/L, ketogenesis started at a range of 1.0-5.0 mmole/L [24]. Watanabe proposed the "basic ketone engine and booster glucose engine" hypothesis from the efficiency of energy production. Still, both βHB and glucose in the fetus seemed to be burned in a dual hybrid engine for energy production like cardiac muscle cells. In spontaneous miscarriage, βHB of the placenta and cord blood was 1.84±0.85 mmole/L and 1.5 mmole/L, respectively, while it was 2.09±0.94 mmole/L and 3.10 mmole/L by artificial abortion. It suggested the low energy production led to miscarriage in the first trimester. βHB as a brain nutrient is well known, as astrocyte is the βHB producer throughout life [25]. Above difference was not present at the second-trimester miscarriage. In mammalian species, glucose and ketone bodies have been considered essential in energy metabolism, but additional brain structure development roles and its smooth function were recently noted. Ketone bodies are crucial for supporting brain development energy and making substrates for biosynthesis for the structural brain beyond the significant energy source in the pregnancy [26]. Rizzo reported that the βHB concentration correlated with neuropsychological offspring's development in the third trimester of pregnancy. They were 223 pregnant women and their singleton offspring: 89 women had pregestational diabetes mellitus, 99 had gestational diabetes mellitus, and 35 had normal carbohydrate metabolism during pregnancy. The associations between gestational ketonemia in the mother and a lower IQ in the child warranted continued efforts to avoid ketoacidosis. However, βHB levels of pregestational diabetes ranged 0.04-0.67 mmole/L in the second trimester and 0.04-0.72 mmole/L in the third trimester. In women with gestational diabetes, βHB was 0.05-0.33 mmole/L in the second trimester and 0.03-0.45 mmole/L in the third trimester. These values did not differ from non-diabetic women, so it is hard to think that the low level of βHB affected the neuropsychological damage. We consider frequent hypoglycemia events (13±13 and 16±12 in the second and third trimester, respectively) in pregestational diabetes, and 0-5 and 0-33 times in the second and third trimester, respectively, would be a cause of brain damage. No data of the first trimester and resultant fetal growth made it difficult to evaluate the real cause of low IQ. The strength of our data is all human data of 600 cases. So, it would be a reference of ketone values in normal pregnancy. Our study's weakness is data limitation, focusing on βHB only with insufficient glucose measurement and other biological markers.
CONCLUSIONS
In fetal life, the placenta produced βHB from the first trimester for energy at a range of 0.4-4.5 mmole/L, and cord blood, ranging from 0.3-3.1 mmole/L. The concentration was the highest in the 2nd trimester and decreased toward the delivery. These are the physiological range, and no harmful effects happened. Fetal energy production depends on glucose and βHB, and the mother's accumulated lipid and fatty acid was a source of lipid oxidation. βHB had many other functions for the development of the fetus.
DECLARATIONS
Funding: The authors have no official fund concerning this research. The APC was funded by T.M.
Conflict of Interest: All authors declare no conflict of interest.
Ethics approval: Ethical Guidelines for Epidemiology Research proposed by the Ministry of Education, Culture, Sports, Science, and Technology, and the Ministry of Health, Labor, and Welfare were also applied. The Ethical Committee approved the research plan in the Life science Promoting Association (#2016-3) and the Ethical Committee for the Clinical Research in the Muneta Maternal Clinic. All mothers who participated to the study were mentally cared if someone had complaints.
Consent to participate:All authors had the annual meeting on low carbohydrate dietary therapy.
Consent for publication: All authors have read and agreed to the published version of the manuscript.
Availability of data: Request to the corresponding author.
Code availability:No particular code.
Authors’ contribution: All the authors have made substantial contributions to the analysis and interpretation of the data in this study. Conceptualization: T.M, S.W.; methodology:Y.N., H.B, K.E.; formal analysis: S.W.; investigation: T.M., M.H.; resources: T.M.; data curation: S.W., H, W.; writing-original draft: H.B., K.E.; writing-review and editing: S.W. H.W.; funding acquisition: T.M.; project administration: T.M.
ACKNOWLEDGMENT
The authors would like to express our gratitude to all related staff to understand and cooperate in the research's progress.
- American Diabetes Association (2020) Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes. Diabetes Care 1: 98‐
- Japan Diabetes Association (2013) Diabetes Clinical Practice Guidelines Based on Scientific Evidence.
- Muneta T, Kawaguchi E, Hayashi M, Bando H, Ebe K (2019) Normalized glucose variability by low carbohydrate diet LCD in CGM study. Asp Biomed Clin Case Rep 2: 22-27.
- Ebe K, Ebe Y, Yokota S, Matsumoto T, Hashimoto M (2004) Low carbohydrate diet LCD treated for three cases as diabetic diet therapy. Kyoto Med Assoc J 51: 125-129.
- Bando H, Ebe K, Muneta T (2017) Clinical Effect of Low Carbohydrate Diet LCD Case Report. Diabetes Case Rep 2: 124.
- Meng Y, Bai H, Wang S (2017) Efficacy of low carbohydrate diet for type 2 diabetes mellitus management A systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract 131: 124-131.
- Rodwell VW, Bender D, Botham KM, Kennelly PJ (2018) Weil PA Harpers Illustrated Biochemistry 31st edn a Lange medical book McGraw Hill 983: 794.
- Polin WW, Fox, Abman SH (2011) Fetal and Neonatal Physiology Elsevier Saunders Philadelphia Pennsylvania.
- Freemark (2006) Regulation of maternal metabolism by pituitary and placental hormones roles in fetal development and metabolic programming. Horm Res 65: 41-49.
- Zeng Z, Liu F, Li S (2017) Metabolic adaptations in pregnancy: A Review. Ann Nutr Metab 70: 59-65.
- Newman JC, Verdin E (2014) Ketone bodies as signaling metabolites. Trend Endocrinol Metabol 25: 42-52.
- Bronisz A, Ozorowski M, Hagner-Derengowska M (2018) Pregnancy ketonemia and development of the fetal central nervous system. Int J Endocrinol 2018: 1242901.
- Cahill GF Jr (2006) Fuel metabolism in starvation. Ann Rev Nutr 26: 1-22.
- Watanabe S, Hirakawa A, Aoe S (2016) Basic ketone engine and booster glucose engine for energy production. Diabetes Res Open J 2: 14-23.
- Hirakawa A, Watanabe S, Tanaka H (2015) Kodas fasting therapy Energy balance and intestinal bacteria flora. Adv Food Tech Nutr Sci Open J 1: 112-123.
- Watanabe S, Hirakawa A, Utada I (2017) Ketone body production and excretion during wellness fasting. Diabetes Res Open J 3:1-8.
- Muneta T, Kawaguchi E, Hayashi M (2019) Clinical effect for diabetic pregnant female by low carbohydrate diet LCD and continuous glucose monitoring CGM. Obstet Gynecol Int J 10: 326-329.
- Muneta T, Kawaguchi E, Nagai Y (2016) Ketone body elevation in placenta umbilical cord newborn and mother in normal delivery. Glycation Stress Res 3: 133-140.
- Strauss JF, Mesiano SA (2020) Placental Production of Peptide Steroid and Lipid Hormones. J Matern Fetal Neonatal Med 2020: 685-706.
- Lienermann M, Marks AD (2013) Marks Basic Medical Biochemistry. Lippincott Williams and Wilkins Philadelphia Pennsylvania.
- Abbott Co Ltd Owners Guide (2021) Precision Xtra. Available online at: https://www.abbottdiabetescare.com/precisionxtra#nogo
- IBM SPSS subscription (2020) Ver 25.0 Tokyo Japan.
- Steiner P (2019) Brain Fuel utilization in the developing brain. Ann Nutr Metab 75(suppl 1): 8-18.
- Goyal MS, Iannotti LL, Raichle ME (2018) Brain Nutrition A life span approach. Ann Rev Nutr 38: 381-399.
- Puchalska P, Crawford PA (2017) Multi-dimensional roles of ketone bodies in fuel metabolism signaling and therapeutics. Cell Metab 25: 262-284.
- Rizzo T, Metzger BE, Burns WJ, Burns K (1991) Correlations between antepartum maternal metabolism and intelligence of offspring. N Engl J Med 325: 911-916.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Neurosurgery Imaging and Techniques (ISSN:2473-1943)
- Journal of Oral Health and Dentistry (ISSN: 2638-499X)
- Advance Research on Endocrinology and Metabolism (ISSN: 2689-8209)
- International Journal of Internal Medicine and Geriatrics (ISSN: 2689-7687)
- Journal of Cancer Science and Treatment (ISSN:2641-7472)
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)
- BioMed Research Journal (ISSN:2578-8892)






