Research Article
Risks, Expansion and Regulation of Clinical Trials of Regenerative Medicine in Brazil: An Overview
4171
Views & Citations3171
Likes & Shares
Regenerative medicine, which encompasses cellular and gene therapies and tissue engineering, has changed the conventional medical paradigm. Globally, this expanding sector is at the stage of clinical trial development, product manufacturing, and gradual adoption of products by the health system. But a series of significant scientific, technical, ethical, and regulatory lacunae and contradictions continue to impact progress, with each country implementing solutions of its own. This article, a qualitative and statistical study, illustrates the principal characteristics and regulation of recent clinical trials in RM in Brazil. In spite of the unresolved issues in RM, clinical trials are expanding locally, although only one product has been granted market approval. This process has been facilitated by very recent regulation that has introduced flexibility in trial evaluation, implementation, and product approval. It is expected that in the near future new initiatives regarding trials will multiply significantly. Regulatory decisions follow, to some extent, frameworks for advanced medicinal products in Europe and the USA, and then are adapted to local standards and needs, implementing what some authors have defined as national “home-keeping policies.” These are designed by RM leaders when countries respond to universal standards, but do not contribute to local planning of economic, scientific, and health development. Furthermore, new local capacities for multicenter international trials, the upgrading of capabilities in the public health system, and the creation of adequate infrastructure, especially related to the manufacturing of allogeneic products, need to be further developed for the Brazilian sector to flourish. Finally, for the new scientific and medical framework to be better understood and socially accepted, there is a need for a wider public debate in Brazil on topics specific to RM.
Keywords: Advanced therapy, Cellular therapy, Regenerative medicine, Stem cell clinical trials, Clinical trials
INTRODUCTION
Regenerative Medicine (RM) is a new expanding sector that comprises cellular and gene therapies as well as tissue engineering. These therapies, underpinned by the core principles of rejuvenation, regeneration and replacement, are shifting the paradigm in healthcare from symptomatic to curative treatment [1].
Global RM is in the stage of clinical trial development, product manufacturing, and gradual market adoption of products. Yet the translation of products into daily health practices remains in its infancy [2]. At the present time, there are still important scientific, technical, ethical and regulatory lacunae that impact progress [3]. The resolution of these aspects varies across countries according to the “home-keeping” policies implemented - to be explained below.
MATERIALS AND METHODS
The main aim of this article is to illustrate the principal characteristics and regulation of clinical trials in cellular therapy (CT) in Brazil over the past decade. It is a qualitative and quantitative study based upon bibliographic and documentary sources as well as the analysis of available statistics. The study focuses on answering the following questions: Which are the main risks and uncertainties of these therapies at the global level? What types of clinical trials of CT are being developed in Brazil? How can the national regulation and product approval process be described?
For this purpose, information on risks, regulation, and clinical trials was gathered in the following stages:
- The archives of national and international regulatory agencies were searched and journalistic reports were analyzed to identify clinical trials and their regulation.
- Statistical data on CT clinical studies for the last decade were collected from the ClinicalTrials.gov platform of the National Institutes of Health (NIH) and contrasted with the corresponding data available on the national platform of the Brazilian Registry of Clinical Trials (REBEC; www.rebec.gov.br).
- Fifteen interviews conducted with the RM leadership in Brazil and the responses were analyzed.
TECHNOSCIENTIFIC, ETHICAL AND ECONOMIC RISKS
One of the principal challenges faced by RM is making the transition from a conceptual laboratory research model and the clinical trials of the initial phases toward the production of standardized therapies on a scale that allows for the reduction of treatment costs. A major obstacle in scaling up is the lack, at the global level, of specific manufacturing procedures for these products. Figure 1 illustrates the complexity of manufacturing flows for allogenous CT, where donor and receptor differ, and autologous CT, where they coincide. Risks and uncertainties are common at all manufacturing stages.
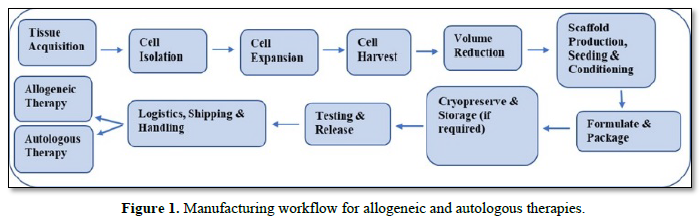
Presently the sector is dominated by clinical trials of autologous CT, with cells produced in small batches at the research site. Clinical work involves small patient cohorts with diseases at different stages of progression. This practice generates a great variety of results that are difficult to compare.
RM treatment costs tend to be too high and the risks/uncertainties, which include (a) the sources of biological materials, (b) the manipulation of cells and genes, (c) the application stage, and (d) the environment [4], little known.
- Sources: The variability of biological materials - stem cells’ potential for differentiation and proliferation - can present oncological risks for patients via cell migration to unexpected body sites of the cells injected or in vivo differentiation of into other type of cells. Multiple risks arise from the scarcity of standardization protocols for cell and tissue characterization, isolation methods, cultures, cell purification and expansion, input processing in insufficiently isolated spaces, and in the quality control of intermediary and final products. Variability also affects the measurement of parameters of potential deterioration, infection, toxicity, duration, and viral and microbial infection in the inputs deployed.
- Manipulation: Inadequate handling techniques can add risk, if cells/tissues are exposed to mechanical and oxidation stresses. Different degrees of cell manipulation (‘minimum’ and ‘more than minimum’) should not interfere with cellular viability and instability or produce genotype alterations among receptors. Risk management should be extended to the various production sites including institutions such as cell and tissue banks and innovation accelerator agencies.
- Applications: Fresh cells might need to be administered to patients a few hours after being collected; cryopreservation and storage might also produce cell deterioration. Minor surgery, such as bone marrow aspirations or skin/muscle biopsy, is usually required to develop products, except in the heart biopsies where improvements in one part of the body may harm another. The persistence of products in the human body after therapy application and their prolonged effects and potential toxicity make it impossible to recruit healthy volunteers for phase I clinical trials. Patients are only being recruited for trials classified as phase I/II. In the case of allogenous treatments, the potential for adverse immunological responses is to be estimated before therapy application. In the case of gene therapy, which uses viruses or nanotechnologies as transport vectors for cell products, new control parameters (e.g., transduction, purification, and microfiltration) must be considered [5].
- Environment: The characteristics of the laboratory environment, infrastructure, and transport can also affect CT’s healing properties. Cell culture within open spaces in laboratories can be affected by temperature, pH, and humidity. Waste from scientific and productive processes must be treated to prevent environmental contamination. Besides following good clinical and manufacturing practices, health risks can be controlled through two other paths, social governance and individual consent. The first is based on a wider reference system of culturally shared standards and values across institutions and publics [6]. There is an international consensus on the need to scrutinize these technologies ‘upstream’ as well as on negotiating agreements on acceptable levels of uncertainty in relation to the therapies’ clinical efficacy and cost-effective use [7,8]. This positioning can contribute towards incentivizing a wider public dialogue on CT to establish risk management strategies pre- and post-market and anticipatory governance.
The second path is made more difficult by scientific and medical experimentation facing many unknowns. For example, donors might not be able to foresee how the cells collected for a specific study will be used in the future, giving rise to debates on the ethical validity of donations with wide consent; in addition, conflicting visions of the control of stem cell lines abound [9].
The intertwined ethical and economic questions associated with RM can be analyzed in relation to four main criteria: equity, reimbursements, cost efficiency of therapeutic options, and patents. Public policies should consider whether to prioritize investment in advanced therapy development at a higher cost, inevitable in a cutting-edge infant industry, vis-à-vis investment in lower-cost conventional therapies. However, state intervention in the market to promote equity - through the public health system and/or the mandatory control of private health plans - may coexist with institutions with authority over selective reimbursement of therapies.
Public reimbursement of therapies can be supported by several policy instruments:
- Innovation incentives from health care cost reimbursement agencies
- Special rules for rare or orphan diseases: patent protection, financing of initial research, subsidies, and tax reduction
- Funding of ‘end of life’ or incurable disease treatments, as is usually demanded by patient associations.
Differential levels of involvement across countries in the international patent system also influence the field. Patents in RM have so far been concentrated in processes and equipment, largely due to the dominance of autologous treatments in situ [16]. But this situation may change significantly with the recent entry of large pharmaceutical firms into the sector and market approval of a larger number of allogeneic products.
RESULTS AND DISCUSSION
The regulation of clinical trials in Brazil
In Brazil, ethical evaluation of clinical trials is carried out by the National Commission on Ethics in Research (Conep), which approves therapy projects based on genes and other human biological materials, in tandem with its associated research ethics committees (CEPs) from universities and hospitals. The National Agency of Sanitary Vigilance (Anvisa) is involved in the technical evaluation of projects. In the past, these entities have been widely criticized by the scientific community and clinical trial sponsors due to the long delays that hinder development of new clinical studies [17].
It took a long time for the regulatory process of RM clinical trials to mature in Brazil. The design of regulations gradually integrated similar clauses to those in the European and American frameworks. In 2004, Anvisa published the Resolution of the Collegiate Board (RDC) 219 to regulate all clinical studies, allow their outsourcing to specialized firms, and present guidelines on good clinical practice. The RDC 39 of 2008 allowed simultaneous evaluation by Anvisa and the research ethics committee of the coordinating center. In 2012, RDC 36 21 superseded RDC 39 in order to simplify the analysis of studies already approved by a relevant international regulatory agency. Registration of clinical trials in the Brazilian Registry of Clinical Trials (REBEC) became mandatory, which is part of the World Health Organization International Clinical Trials Registration Platform (ICTRP/WHO). Issued in 2013, RDC 38 regulated medicines within the program for “patients’ expanded access,” compassionate use, and posttrial delivery.
Through the RDC 9 24 of 2015, evaluation of all clinical trials related to a specific medicine/therapy became centralized, following the recommendations of the International Conference on Harmonization (ICH). From then on, the time frame for evaluation was reduced by five months on average compared to 2013, as stated in the interviews conducted.
For gene, cellular therapy, and tissue-engineering products, Anvisa adopted the name endorsed by the European Medicines Agency (EMA): advanced therapy medicinal product (ATMP). It published three new regulatory resolutions:
- RDC 214 of February 2018 reports on good clinical practices in human research using clinical-grade cells
- RDC 260 of December 2018 discusses procedures for research-level clinical trials in advanced therapies
- RDC 338 of January 2020 establishes minimum requirements for the approval of these products for use and commercialization within Brazil.
This resolution also makes obtaining further authorization from the National Technical Commission on Biosecurity (CTNBio) when the trials involve a genetically modified organism a requirement.
These products are classified as either
- ATMP class I, those undergoing “minimum manipulation,” which does not significantly alter their biological characteristics (differentiation and activation states, potential proliferation, and metabolic activity)
- ATMP class II, those undergoing “extensive manipulation,” which can alter any of these.
For each product category, protocols and documentary requirements differ regarding, for example, dosage level; toxicity; impurities; interaction with other tissues; immunogenic effects; tumor potential; donor and human material initial selection; reporting on scaffolds, matrixes, and devices used; and manufacturing procedures. Registration is initially valid for 5 years. After the first registration renewal of products in class I and after the second renewal of class II products, an extension of 10 years can be requested. (Stem cell therapies are class II products, as they require ex vivo cell culture before therapeutic application).
This last resolution by Anvisa makes room for the clinical application of biological therapies in hospitals and consulting rooms. Any medical doctor trained to perform a specific treatment can apply it, without being linked to any ongoing clinical trial. The Director of Blood, Tissue, Cells and Organs (GSTCO) of Anvisa publicly declared that if a patient has run out of therapeutic options, he/she may be authorized to undergo an advanced therapy treatment. If Anvisa’s evaluation is incomplete over a 30-day time span, the medical doctor is automatically authorized to perform the treatment anyhow. Patients will be followed up closely by the agency during 5 years and therapy revalidation will depend on efficacy [18].
The new regulation considerably simplifies the regulatory burden for researchers and introduces flexibility into trial development, in keeping with the global trend. Early in the last decade, leading agencies in RM took several steps toward the international harmonization of regulations, e.g., of policies from the International Society for Stem Cell Research [19]. Nowadays, regulatory diversification predominates and is supported by specific scientific communities represented by organizations like the International Society for Cellular Therapy (ISCT). Diversification is based on “bio entrepreneurial ship,” “bio networking,” and “international entrepreneurial ship in the biosciences,” which coordinate RM activities and local methods with international contacts [20,21]. These processes are conditioned by national “home-keeping” policies. Scholars contend this heuristic notion accounts for the design of public policies by a country’s RM leaders when aligning with universal standards and for the resistance that follows because the policies do not support local economic, scientific, and health development [22]. Flexible decision-making processes within the regulatory landscape are at the intersection between the local and international levels of regulation - alternatively operating with “soft” (ethical) or “hard” (mandatory) rules. The recent situation in Brazil on RM somewhat reflects these trends towards an implementation of national “housekeeping” policies.
Clinical trials in cellular therapy and approved RM products in Brazil
The registration of CT clinical trials in Brazil presents contradictions between different sources for the period 2010-2020. The international platforms of clinical trials of the US National Institutes of Health (NIH) and the Brazilian Registry of Clinical Trials (REBEC) were consulted to establish comparisons.
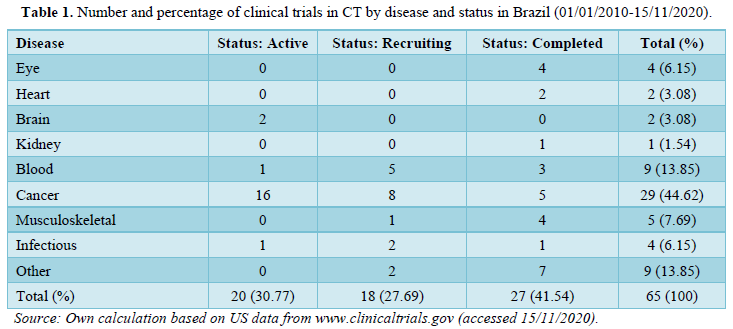
In the ClinicalTrials.gov database, using the keywords “stem cells” or “cell therapy,” 65 studies were found between 01/01/2010 and 15/11/2020. These represent only 1.62% of those at the global level for the last decade. Almost one half of the Brazilian trials deal with cancer (29 cases, 44.62%), with the next largest category being blood diseases (9 cases, 13.85%). The rest comprise a variety of health conditions, especially related to musculoskeletal and eye disease (5 and 4 trials, respectively) (Table 1). Though a considerable number of trials have already been completed (41.5%), more than half are still active or in the recruitment stage.
Other data show that trials are evenly distributed between those considered international - sponsored by a foreign institution - and local ones, where sponsors involve one or more institutions from the same state in Brazil. But while the vast majority of the international studies are ongoing (29 cases), most local studies (24 cases) have already been completed, pointing to the recent entry of foreign capital into the local sector. The lack of national trials, partly reflects the origin of the funding - e.g., by state research agencies-and partly that organizers target local participation to facilitate access and follow-up with patients.
Information from the same platform complemented with internet research shows the behavior of international private capital, which sponsors approximately two-thirds of Brazilian
CT clinical trials, mostly in phase III. Big pharma - a recent entrant into the sector - is represented by 10 of the major global firms developing 21 clinical trials (32.30% of the total). Millennium Pharmaceuticals has undertaken the highest number of trials (5), followed by Novartis (4) and Janssen Research & Development, LLC, of Johnson & Johnson (4). A smaller group of studies (5) is sponsored by international biotechnology firms, generally represented by medium-sized firms specializing in advanced therapy, such as Gamida Cell Ltd, Amgen, Celgene, ReViral, and Genentech. These firms usually also access donations from specific patients or patient organizations.
The rest of the clinical trials registered are sponsored primarily by public institutions (16 studies, 24.6%) and secondarily by national private ones (10 trials, 15.38%). They are usually implemented at universities, research centers, and hospitals. It is likely some of those trials are in partnership with international firms, though the platform’s data are unclear in this respect.
Among public institutions, the largest number of trials is undertaken by the University of São Paulo (USP) (10) - over a third of public studies - followed by the University of Rio de Janeiro (UFRJ) (3) and the Federal University of São Paulo (UNIFESP) (3). Among national private institutions, various trials are carried out by the São Rafael Hospital (3) in Salvador, State of Bahia; and the Israelite Hospital Albert Einstein (3) and the Syrian-Lebanese Hospital, both in the State of São Paulo (2). Atypically, two individuals are sponsoring two different phase I trials: a member of the Board of the Brazilian Association of Cellular and Gene Therapy (ABTcel) - a very active organization founded by the local scientific community - and a medical doctor from the National Institute of Cancer (INCA).
Other data in this platform show that more than a third of clinical trials (23 cases, 35.28%) are phase III, either active (13) or in the recruitment stage (6). They focus on cancer therapies and blood disease. Phase II studies are in second place (14 cases, 21.54%), evenly divided between ongoing and completed.
A total of 15,406 subjects are participating in these trials, with more than half having been recruited for the 23 ongoing phase III trials (7,693 patients) (Table 2). Phase II trials involve 1,595 patients, approximately half of whom have participated in previous studies. In Brazil, then, the number of subjects in ongoing phase II and III studies has grown significantly.
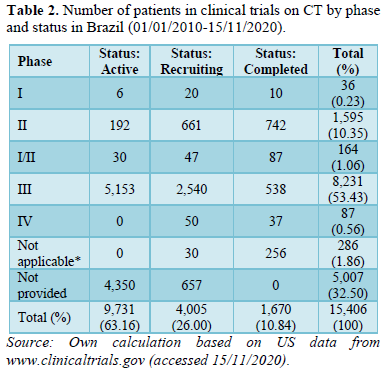

Note: *Refers to studies that do not specify their phase following the US Food and Drug Administration (FDA) categories, including those of medical devices and behavioral interventions.
However, it is striking that there has been follow-up with only 87 patients via phase IV trials, i.e., post product approval. It is likely that these patients were treated with therapies with a longer tradition in Brazil, like bone marrow blood transplants, as no other CT had been locally approved until very recently. Unfortunately, for almost a third of the universe of patients enrolled in registered active trials - a total of 5,007 participants - the phase is not indicated.
In REBEC, again using the keywords “cell therapy” or “stem cells” for the period under study, a total of only 22 registered trials was found whose characteristics will be summarized next. These trials dealt with totally different pathologies from those registered on the platform analyzed above, including respiratory, hepatic, skin, urinary, musculoskeletal, and inflammatory/infectious diseases.
Yearly increases in the number of studies - measured by the initial recruitment date - have been constant since 2010. Trials are concentrated in the year 2018 (7 studies) and tend to be of a medium duration, averaging between a year and a half and two years. However, a quarter of the studies (6) will last just a few months and maybe phase I.
Five of the trials registered are recruiting in foreign countries: the USA, the United Kingdom, and Vietnam. They have been developed by large pharmaceutical firms such as GlaxoSmith-Kline and Bristol-Myers Squibb and usually relate to rare diseases. These international sponsors are conducting “preventive” registration in Brazil; they aim to expand patient recruitment in the local market, if needed in the future.
A little over 40% of the trials are taking place at and are underwritten by public universities and hospitals (9), and in general involve only 1-2 research centers; 5 trials are carried out by private hospitals. In the former group, similarly to the results from the other registry consulted, USP, UFRJ, and UNIFESP are conducting most of the trials, and in the latter, the Syrian-Lebanese and the Albert Einstein Hospitals, institutions in the Southeast Region of Brazil, are conducting the most. But some trials have also been sponsored by regional hospitals in the southern states, like Paraná and Porto Alegre. Just one study is sponsored by a charity, the Israelite Brazilian Charity Society, and one by a private research center, the Camargo Cancer Center.
In the rest, domestic private capital tends to participate as sponsor in partnership with international capital. There are two exceptions, the cases of the national health operator Prevent Senior and of the domestic firm Stemcorp, a spin-off from USP that is financing two studies. The latter firm specializes in the isolation, multiplication, and storage of mesenchymal stem cells from the umbilical cord and placenta blood, adipose tissue, and tooth pulp and figures prominently among the very few local start-ups in the sector. Only one large pharmaceutical firm, REGENXBIO Inc., recruits local subject for one trial.
More than half of the trials are at the recruitment stage (13), with 5 being at the organizational stage. Only one clinical study has finished recruitment and another completed data analysis. This confirms that more than half of the studies are very recent, possibly due to the impact on the sector of Anvisa’s last resolution that, according to the local RM researchers interviewed, incentivized the expansion of the local sector into clinical translation.
Conclusive comparisons of data results between both platforms analyzed cannot be made. In general, though, the diversification of the registered clinical trials reflects the specific preferences and interests of different members of the local scientific and medical community. Those researchers who have regularized their technical and bioethical research procedures and want the eventual approval of the products resulting from their trials tend to publish in REBEC, the mandatory registry. Interviews carried out with 15 Brazilian leaders in RM during previous and ongoing research projects corroborate this argument. It should be recalled that the Clinicaltrials.gov registry does not require that clinical trials, prior to publication, be granted any technical or ethical authorization [4,5,11,19,16,23].
At the same time, a substantial number of clinical trials in Brazil have not been registered on any platform, according to our interviews with the RM leadership. For example, a recent partial report by INCT-REGENERA at the Federal University of Rio de Janeiro (UFRJ), one of the main centers of research in RM, lists numerous phase I clinical trials for treatments of 10 different diseases [13], with the authors’ mentioning that these trials were carried out “independently from the INCT” and not offering further explanation.
Partly as a result of the regulatory changes mentioned above, on October 6, 2020, Anvisa approved the first genetic therapy in Brazil, a treatment that had been recently authorized in Europe and the USA. The advanced therapy, Luxturna, is produced by the American firm Spark Therapeutics and is used for children 12 months old up to adults in the treatment of hereditary retina dystrophy [24].
CONCLUSIONS
On the one hand, due to Anvisa’s normative resolution RC 383 of 2020 and local advances in basic and clinical RM research, it is to be expected that a much larger number of clinical trials will be carried out in Brazil in the near future and be registered at the REBEC database. Also, the rich ongoing phase II and III clinical experimentation documented in this paper will most likely become an important step toward local approval, in the short term, of a greater number of RM products.
On the other hand, regulatory flexibility regarding the administration of these therapies in medical consulting rooms - as part of national “home-keeping” policies - could increase risks and the dispersal of ‘hard to monitor’ clinical initiatives. For RM to be better understood and socially accepted, wider public debate is required. Inclusive social dialogue about clinical advancement in the sector has been long absent in Brazil, as has regular quality news reporting in the mass media [25].
The feasibility of the wide application of allogeneic therapies depends upon conditions that are rarely met in Latin American countries [26]. New innovation platforms/hubs and industrial manufacture and adequate transport systems need to be designed and hospital infrastructure and handling capability upgraded, based on new standards of purification, storage, and of security and decontamination of biological products.
Furthermore, substantive financial resources, limited in Brazil, are needed to carry out large-scale phase III trials. Along these lines, local capacity for extensive and symmetric international collaboration in multicentric trials has to be improved, as is being discussed globally [14,27,28]. The diffusion of RM in the public health system will require the involvement of specialized actors: medical and digital specialists, surgeons, technicians, nurses, and administrative personnel who must be trained for this specific purpose [12]. However, the devotion of financial and human resources exclusively to basic research on stem cells would make Brazil dependent on imports of materials, equipment, and off-the-shelf advanced therapies and make the adoption of advanced therapies by SUS even more expensive [8,29].
- Jessop ZM, Al-Sabah A, Francis W, Whitaker I (2016) Transforming healthcare through regenerative medicine. BMC Med 14: 115.
- Hunsberger J, Harrysson A, Shirwaiker R, Starly B, Wysk R, et al. (2015) Manufacturing Road Map for Tissue Engineering and Regenerative Medicine Technologies. Stem Cells Transl Med 4: 130-135.
- McMahon DS, Thorsteinsdottir H (2013) Pursuing endogenous high-tech innovation in developing countries: A look at regenerative medicine innovations in Brazil, China and India. Res Policy 42: 965-974.
- Gardner J, Higham R, Faulkner A, Webster A (2017) Promissory identities: Sociotechnical representations & innovation in regenerative medicine. Soc Studies Sci 174: 70-78.
- Foley L, Hicks C, O´Neill P (2011) Supply chain management in regenerative medicine manufacturing. In: Spath D ed. 21st International Conference on Production Research: Innovations in Product and Production. Stuttgart: Fraunhofer IRB Verlag.
- Brown N, Beynon-Jones SM (2012) Reflex regulation: An anatomy of promissory science governance. Health Risk Soc 14: 223-240.
- Wilsdon J, Willis R (2004) See-through science: Why public engagement needs to move upstream: London: Demos.
- Zatz M (2011) Genetics: Choices that our grandmothers didn't make. São Paulo: Editora Globo.
- Skloot R (2010) The immortal life of Henrietta Lacks. New York, NY: Crown Publishers.
- Bubela T, McCabe C (2013) Value-engineered translation for regenerative medicine: Meeting the needs of health systems. Stem Cells Dev 22 (suppl 1): 89-93.
- Eisendel EF, Adamson H (2012) Stem cell tourism and future stem cell tourists: policy and ethical implications. Dev World Bioeth 12(1): 35-44.
- House of Commons Science and Technology Committee (2017) Regenerative medicine. Fifteenth Report of Session 2016-2017. London: House of Commons.
- Inct-Regenera (2019) Partial report by the National Institute of Science and Technology in Regenerative Medicine (INCT-REGENERA). Accessed on: October 15, 2020. Available online at: https://www.inctregenera.org.br/uploads/5/4/0/8/5408654/relatorio_t%C3%A9cnico_parcial_inct-regenera_20193098.pdf
- Kleiderman E, Boily A, Hasilo C, Knoppers BM (2018) Overcoming barriers to facilitate the regulation of multi-centre regenerative medicine clinical trials. Stem Cell Res Ther 9: 307.
- McMahon DS (2014) The global industry for unproven stem cell interventions and stem cell tourism. Tissue Eng Regen Med 11(1): 1-9.
- Davey S, Davey N, Gu Q, Xu N, Vasta R, et al. (2015) Interfacing of science, medicine and law: The stem cell patent controversy in the United States and the European Union. Front Cell Dev Biol 3: 71.
- Acero L (2011) Stem Cell Research and Therapies: Social Views and Debate in Brazil. Rio de Janeiro: E-Papers.
- Saad N (2020) In an unprecedented decision, Anvisa releases alternative therapies in Brazil (21/02/2020). Accessed on: November 15, 2020. Available online at: https://noticias.r7.com/saude/em-decisao-inedita-anvisa-libera-terapias-alternativas-no-brasil-21022020?amp
- Daley GQ, Hyun I, Apperley JF, Baker R, Benvenisty N, et al. (2016) Setting global standards for stem cell research and clinical translation: The 2016 ISSCR guidelines. Stem Cell Rep 6: 787-797.
- Sleeboom-Faulkner M, Patra PK (2011) Experimental stem cell therapy: Bio hierarchies and bio networking in Japan and India. Soc Stud Sci 41(5): 645-666.
- Rosemann A, Bortz G, Vasen F, Sleeboom-Faulkner M (2016) Global regulatory developments for clinical stem cell research: Diversification and challenges to collaborations. Regen Med 11(7): 647-657.
- Sleeboom-Faulkner M, Chekar C, Faulkner A, Heitmeyer C, Marouda M, et al. (2016) Comparing national home-keeping and the regulation of translational stem cell applications: An international perspective. Soc Sci Med 153: 240-249.
- Cossu G, Birchwall M, Brown T, De Coppi P, Culme-Seympur E, et al. (2018) Lancet Commission: Stem cells and regenerative medicine. Lancet Commissions 391(10123): 883-910.
- Reis F (2020) CTNBio approves Luxturna - gene therapy against blindness. Accessed on: November 10, 2020. Available online at: https://pfarma.com.br/noticia-setor-farmaceutico/mercado/5208-ctnbio-aprova-luxturna-terapia-genetica-contra-cegueira.html
- Acero L (2020) News on regenerative medicine in Brazil: The quality of press reports. J Genet Cell Biol 3(2): 162-168.
- Bortz G, Rosemann, A, Vasen F (2019) Construction of stem cell therapies in Argentina: Regulation, risk management and innovation policies. Sociologies 21(50): 116-155.
- Sipp D, Sleeboom-Faulkner M (2019) Downgrading of regulation in regenerative medicine. Science 365(6454): 644-646.
- Rosemann A (2015) Multi-country stem cell trials: The need for an international support structure. Stem Cell Res 14: 396-400.
- Rehen S, Paulsen B (2007) Stem cells: What are they? What are worth for? Rio de Janeiro: Vieira & Lent.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Genomic Medicine and Pharmacogenomics (ISSN:2474-4670)
- Journal of Agriculture and Forest Meteorology Research (ISSN:2642-0449)
- Journal of Astronomy and Space Research
- Journal of Microbiology and Microbial Infections (ISSN: 2689-7660)
- Journal of Biochemistry and Molecular Medicine (ISSN:2641-6948)
- Journal of Womens Health and Safety Research (ISSN:2577-1388)
- Advances in Nanomedicine and Nanotechnology Research (ISSN: 2688-5476)



