Research Article
Safety and Efficacy of Autologous Adipose Derived Mesenchymal Stem Cells (ADMSCS) Transplantation
4008
Views & Citations3008
Likes & Shares
Methods: We have lipoaspirated adipose tissue from lower abdomen of 52 patients and isolated ADMSCs using density gradient centrifugation. The characterization of ADMSCs was done using positive and negative i.e., immunohistochemical markers. 52 patients with nonhematopoietic degenerative conditions including 26 with osteoarthritis. They were treated with ADMSCs. The route of administration was Intrathecal/IV injections/intraspinal for degenerative neurological conditions dosage 2-4 million cells/kg body weight. For osteoarthritis knee, the route of administration was intraarticular.
Results: The hematological and biochemical parameters showed no major deviation from the normal. Clinically, no acute adverse effects or Graft Versus Host Disease were observed with the dose used. For neurological conditions, there was a variable improvement from 20% to 95%. In osteoarthritis knee, the efficacy was 90%.
Conclusion: This study supports clinical safety and potential therapeutic medical use of ADMSCs. It did not require immuno-suppression drugs.
Keywords: Degenerative conditions, Graft-Versus Host Disease (GVHD), Human ADMSCs, Regenerative Medicine, Stem cell Therapy
INTRODUCTION
Stem cell therapy is novel alternative approach in therapeutics because they possess self-renewal capacity. They translate to all three germinal layers [1]. They are not only immune-naïve but are immune-protective as well [2,3]. Mesenchymal stem cells are obtainable from bone marrow, umbilical cord and adipose tissue. They have the same properties. Adipose tissue has an abundant population of MSCs, up to 30%. Additionally, large volumes can be obtained. Subcutaneous adipose tissues are easily available and readily accessible [4]. We therefore focused on ADMSC. They have multilineage potential, i.e. they differentiate into chondrocyte, adipocyte, osteocyte, myocyte, hepatic cells and neuron-like cells and exhibit self-renewability [5,6] MSCs have ability to replace various damaged tissues including cartilage, bone, tendon, vasculature, liver, kidney and nerves.[7] They are able to migrate/hone to the area of inflammation and injury [8] The autologous adipose derived mesenchymal stem cells (ADMSCs) are immunonaive and no risk of GVHD (Graft-Versus- Host-Disease.). The autologous ADMSCs eliminate the risk of transfusion infection and guarantee the cell quality and potency. There are many animal and recent human studies have documented that ADMSCs is therapeutic tool for wide range of degenerative disorders, metabolic disorders like diabetes [9], liver cirrhosisx, xi defect in cartilagexii, neuronal damagexiii, retinal angiogenesis and many more. The present deals with the safety and efficacy with autologous ADMSCs transplantation for potential use in clinical trials. ADMSCs with Platelet Rich Plasma (PRP) are injected intraarticular for treating Osteoarthritis and Rheumatoid Arthritis. Intra-muscular or intravenous for diabetes neuropathy, liver cirrhosis, muscular dystrophy and intrathecal for spinal cord injury, cerebral palsy and supranuclear palsy.
MATERIALS & METHODS
The procurement of adipose tissue and the clinical trial was approved by the Institutional Ethics Committee and Institutional Committee for Stem Cell Research and Therapy, Total Potential Cells (P).
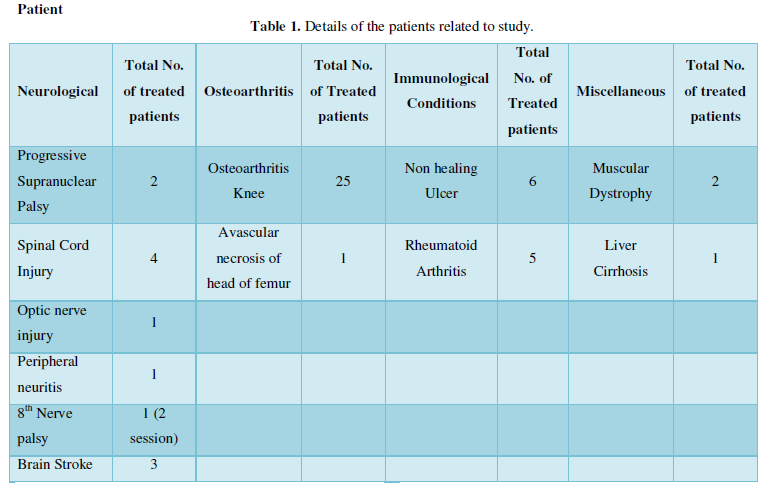
Patient
A written consent from patients was obtained after discussion. The details of the patients related to study are described in Table 1. Patients belong to 4 different categories. Viz. namely neurological, osteoarthritis knee and immunological conditions such as rheumatoid arthritis and non-healing ulcers and miscellaneous.

Inclusion criteria
Patients with age between 10-80 years of either sex was enrolled in the study. Patients with medical examination and evidence for degenerative disease were included for the study. The subjects with metabolic disorders such as hypothyroid were controlled before the initialization of study.
Exclusion criteria
Patients with history of severe allergic reactions, history of malignancies, active infections, sero negative for HIV, HBV, HCV and syphilis, active cardiac, pulmonary, renal, hepatic or gastrointestinal disease; coagulopathy or any other contraindication for lumbar puncture; severe psychiatric disorder were excluded from the study.
MESENCHYMAL STEM CELL PREPARATION
Surgical protocol
Adipose tissue was aspirated by a trained plastic surgeon with specially designed cannulae (Figure 1) under local anesthesia and sedation. Lipoaspirate was collected from lower abdominal subcutaneous fat in the operating room in a sterile container. The samples were transferred to a clean room for cell isolation under strict aseptic conditions.
1.png)
1.png)
Stem cell isolation
The isolation of Mesenchymal Stem Cells was performed with density gradient centrifugation technique. Isolation was performed according to institutional protocol (patent pending). ADMSCs from lipoaspirate were isolated after digestion with 0.2 % collagenase (Gibco Life Technologies, U.S.A, catalogue no: 17100-17) in CO2 Incubator (Thermo Scientific, Forma Series II, Germany) at 370°C with gentle shaking. Cells obtained were made into a pellet at 1500 g in centrifuge (Remi Biotech, Mumbai, India). Cells were further washed twice with PBS and finally with Isolyte M (ClariasOstuka, Ahmedabad, India), and dispensed in 2-3 cc of Isolyte M.
Culture and expansion of ADMSC
Following 2 passages, MSCs were obtained upto 92-95% purity.
Deployment of stromal vascular fraction
SVF that is obtained with the above protocol as the first step was utilized for treating osteoarthritis knee as well as rheumatoid conditions.
Characterization of ADMSCs
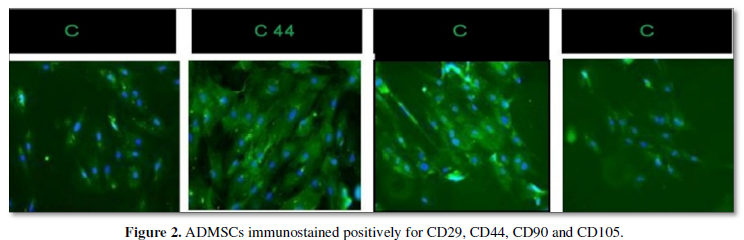
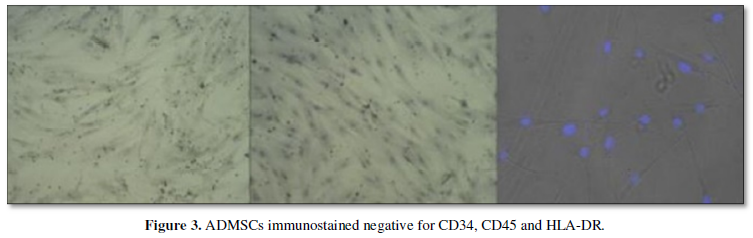
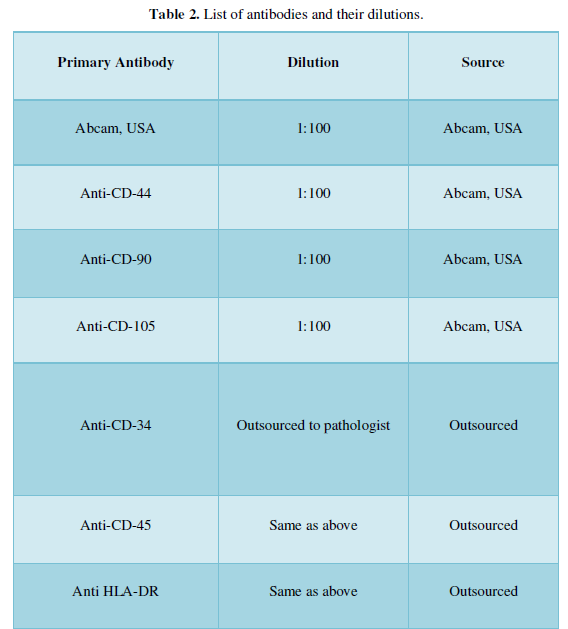
To standardize the protocol for isolation of ADMSCs were subjected to characterization for CD 29, CD 44, CD 90, CD 105 (Figure 2 positive markers) and CD 34, CD 45, HLA-DR (Figure 3 negative markers). Briefly, cells were fixed with freshly prepared 4% Paraformaldehyde (PFA) for 10 minutes at room temperature and permeabilized with 0.1% Triton X-100 for 3-5 min on ice. Primary antibodies were incubated overnight at 4˚C, washed with PBS and then incubated with the secondary antibodies (Donkey anti-rabbit Alexa Flour 488; Jacksons Immunoresearch, United States) at room temperature for 1 h. Slides were washed in PBS and mounted with Vectashield (Vector-Laboratories, CA, United States). DAPI (4,6- diamidoino-2-phenylindole) (Invitrogen, United States) was used to visualize nuclei. The sources of primary and secondary antibodies along with their dilutions are provided in Table 2. Finally, cells were examined, and images were captured using fluorescent microscope. Morphologically, the isolated cells were phenotype of a typical MSCs. Characterization with positive markers CD 29, CD 44, CD 90, CD 105 in Figure 2 and negative markers CD 34, CD 45, and HLA-DR in Figure 3 was successfully done and verified under fluorescent microscope.



ADMSCs administration
Isolated 2-4 million ADMSCs cells/kg of body weight and were then suspended in 2 ml of Isolyte M (ClariasOstuka, Ahmedabad, India). ADMSCs were injected in combination with 3-5 cc of autologous Platelets Rich Plasma (PRP). PRP was obtained by the standard protocol by Graham, 2002. 4 ml PRP was added to the isolated cell suspension. The cells were given to patients by various modes of administration. The SVF cells were freshly injected in each knee joint intraarticularly. The MSCs were administered intramuscular, intravenous and intrathecal. Depending on the age and the body weight of the patient, hydrocortisone, antibiotics, and antihistamines were given prior to ADMSCs administration. The follow up was done on the patients for clinical and biochemical parameters after the treatment.
RESULTS
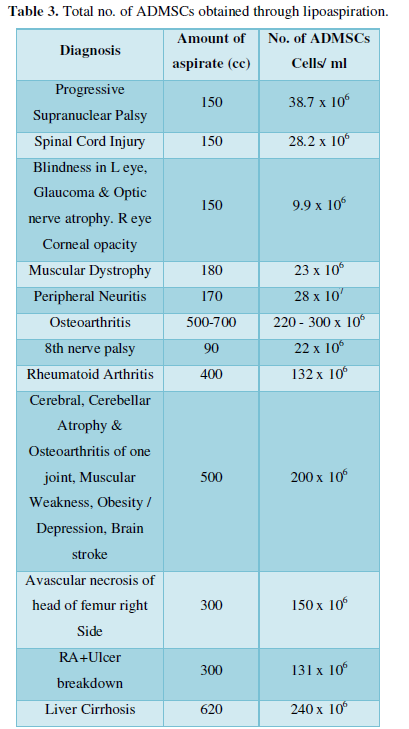
The following Table 3 suggests the amount of aspirate collected from patients suffering from different degenerative conditions.
Adverse events
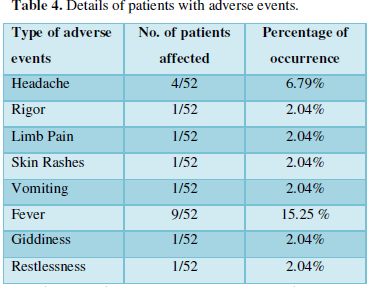
There were no adverse clinical reactions observed; except mild fever for few hours, headache lasting for 1-2 days, rigor lasted for few minutes, skin rashes, giddiness, restlessness.
Their clinical investigations did not suggest any sign of infection and sensitizations. No anaphylaxis occurred. Though used as a precaution once before the administration of ADMSCs cells, anti-allergic drug, antibiotics and corticosteroids were given. Later broad-spectrum antibiotics were continued for 5 days. Only analgesics and anti-pyretic were used in those patients who had fever or headache. No GVHD or serious adverse effects were observed. Table 4 shows the incidence of adverse events in patients after administration of ADMSCs.

Hematological and biochemical parameters remained within normal range. The different parameters such as hemoglobin, total count, platelets count, RBS, WBC, serum creatinine etc. were in standard range. This suggests that no immune response occurred. The values fall in the normal range before and after the treatment and are represented as average range. There were no anaphylactic or allergic manifestations in patients.
DISCUSSION
During last century, molecular substances as a new drug discovery reached its zenith. The impetus was provided by Second World War. Sulphonamides were discovered to be soon followed by the discovery of penicillin by Alexander Fleming. It is due to these drugs that gram positive cocci giving rise to venereal disease such as gonorrhea and syphilis are almost prevented and certainly curable as of now.
However, the cost of a new drug discovery has risen steeply. According to Pfizer, in 2012, a new drug to be certified by FDA was to cost ~$120 million. Within 4 years, the cost has escalated to $2.8 Billion [14]. Therefore, the quest is on to find cheaper modalities for discovering a new drug. Hope is provided through the biologicals and mainly focus is on Mesenchymal Stem Cells. Multinational pharmaceutical giants invest huge amounts for a molecular drug. If a cheaper substitute were to appear, the conflict of interest is obvious. A small group of researchers cannot vie with MNCs. The latter somehow manage to influence and gain control over the regulatory bodies as well. Researchers in this onerous scenario are stretched to utilize their resources to the last drop.

Our data pool therefore is small. Yet, it provides a platform to future researchers to extend their clinical trial to any of the modules discussed herewith.
ADMSCs have many advantages over other sources: availability, no immuno-suppressive drugs, no GVHD and no viral contamination [15,16].
The present study has established the safety and variable efficacy of autologous ADMSCs for regenerative applications. The various routes employed have proven safe. No immune suppressive drugs were given to patients post treatment. This validates that ADMSCs are immune-naïveii. No aggressive intervention was required that establishes that SVF and ADMSCs are entirely safe.
We have established that ADMSCs have potential for trigerminal translation [1]. Therefore, we selected a wide spectrum of neurological conditions [17] as well as osteoarthritis that included age related disorder as well as rheumatoid arthritis that is immunological [18] in origin. The therapy is efficient to treat wide types of injury without sign of any chronic adverse event. The numbers of various disorders treated is small; yet its limited efficacy justifies as a proof of concept to undertake larger studies with highest safe dose.
The limitations to the present study are; no control, the effectiveness of doses at different concentrations was not assessed, the optimal rehabilitation protocol requires to be further investigated, and the sample range for each degenerative condition is very low.
Overall, the results of the present study are encouraging and reinforce the therapeutic interest of ADMSCs for further clinical trials as a treatment option for numerous degenerative conditions.
ACKNOWLEDGMENT
We acknowledge Genome Research Centre for rendering help of confocal microscope for marker studies at M.S. University, Vadodara.
- Vyas B, Shah A, Marathe A, Ansarullah, Vyas R (2018) Adipose tissue: A natural resource for multipotent mesenchymal stem cells with potential translation to trigerminal layers. Indian J Plast Surg 51: 177-181.
- Rawat S, Gupta S, Mohanty S (2019) Mesenchymal stem cells modulate the immune system in developing therapeutic interventions.
- Tyagi R, Bisen P (2019) Immune response activation and immunomodulation.
- Katz AJ, Llull R, Hedrick MH, Futrell JW (1999) Emerging approaches to the tissue engineering of fat. Clin Plast Surg 26: 587-603.
- Graf T (2002) Differentiation plasticity of hematopoietic cells. Blood 99: 3089-3101.
- Watt FM, Oban BM (2000) Out of eden: Stem cells and their niches. Science 287: 1427-1430.
- Aronin CEP, Tuan RS (2010) Therapeutic potential of the immunomodulatory activities of adult mesenchymal stem cells. Birth Defects Res C 90: 67-74.
- Parekkadan B, Mwled JM (2010) Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng 12: 87-117.
- Volarevic V, Rsenijevic N, Uric M, Stojkovic M (2011) Concise review: Mesenchymal stem cell treatment of the complications of diabetes mellitus. Stem Cells 29: 5-10.
- Chang YJ, Liu JW, Lin PC, Sun LY, Peng CW, et al. (2009) Mesenchymal stem cells facilitate recovery from chemically induced liver damage and decrease liver fibrosis. Life Sci 85: 13-14.
- El Ansary M, Abdel Aziz I, S Mogawer S (2012) Phase II Trial: undifferentiated versus differentiated autologous mesenchymal stem cells transplantation in Egyptian patients with CV induced liver cirrhosis. Stem Cell Rev 8: 972-981.
- Bruder SP, Kurth AA, Shea M, Hayes WC, Jaiswal N, et al. (1998) Bone regeneration by Implantation of purified, culture-expanded human mesenchymal stem cells. J Orthopaed Res 16: 155-162.
- Karussis D, Kassis I, Kurkalli BGS, Slavin S (2008) Immunomodulation and neuroprotection with mesenchymal bone marrow stem cells (MSCs): A proposed treatment for multiple sclerosis and other neuroimmunological/neurodegenerative diseases. J Neurol Sci 265: 131-135.
- A DiMasia, Grabowskib HG, Hansenc RW (2016) Innovation in the pharmaceutical industry: New estimates of R&D costs. J Health Econ 47: 20-33.
- Berebichez-Fridman R, Montero-Olvera PR (2018) Sources and clinical applications of mesenchymal stem cells: State-of-the-art review. Sultan Qaboos Univ Med J 18: e264-e277.
- Musiał-Wysocka A, Kot M, Majka M (2019) The pros and cons of mesenchymal stem cell-based therapies. Cell Transplant 28: 801-812.
- Ul Hassan A, Hassan G, Rasool Z (2009) Role of stem cells in treatment of neurological disorder. Int J Health Sci (Qassim) 3: 227-233.
- Rad F, Ghorbani M, Roushandeh AM, Roudkenar MH, et al. (2019) Stem cell-based therapy for autoimmune diseases: Emerging roles of extracellular vesicles. Mol Biol Rep 46: 1533-1549.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Genomic Medicine and Pharmacogenomics (ISSN:2474-4670)
- Journal of Agriculture and Forest Meteorology Research (ISSN:2642-0449)
- Food and Nutrition-Current Research (ISSN:2638-1095)
- Journal of Veterinary and Marine Sciences (ISSN: 2689-7830)
- Journal of Astronomy and Space Research
- Proteomics and Bioinformatics (ISSN:2641-7561)
- Journal of Womens Health and Safety Research (ISSN:2577-1388)





