5980
Views & Citations4980
Likes & Shares
Plants are at the center of Traditional Medicine. Their use in disease management is as old as man [8,9]. Medicinal plants serve as cheap alternative to orthodox medicine since they are readily available [10-12]. Indigenous to West and Central Africa, Icacina trichanta Olav grows in the Savanna areas of Senegal, Gambia, Guinea Bissau, Northern Ghana, Benin and Nigeria [13]. It is a perennial shrub with erect leafy shoot and broad elliptic simple, alternate leaves. In Nigeria, it is known as ibugo in Igbo and Esso gbegbe in Yoruba. Different parts of the plant are used for ethno medicinal purposes [13,14]. Till date, not much is known about the potential of extracts of Icacina trichanta to protect against CCl4-induced cardio toxicity in rats. This study was undertaken to investigate the cardioprotective capacity of aqueous extract of I. trichanta leaves in rats exposed to CCl4.
MATERIALS AND METHODS
Chemicals and Reagents
All reagents used in this study were of analytical grade. Total protein and AST assay kits were products of Randox Laboratories Limited (UK). All other chemicals were obtained from British Drug House (BDH) (England), Merck (Germany) and Sigma-Aldrich Ltd. (USA).
Collection of Plant Material
The leaves of Icacina trichanta were obtained from a forest in Benin City, Nigeria. Its identification and authentication were carried out in the Department of Plant Biology and Biotechnology, University of Benin, Benin City, Edo State, Nigeria. A sample was placed in the herbarium (No: UBHJ 0186). The leaves were then sun-dried, pulverized and sieved.
Plant Extraction
Extraction of the pulverized plant material was by maceration over a 72 h period [15]. A portion (100 g) of the powdered leaf was soaked in 1000 mL distilled water. The resultant aqueous extract was filtered with a muslin cloth and freeze dried using a lyophilizer.
Experimental Rats
A total of 35 rats weighing 160 to 180 g (mean weight = 170 ± 10 g) were obtained from the Department of Anatomy, University of Benin, Benin City, Nigeria. The rats were housed in metal cages under standard laboratory conditions: temperature of 25ºC, 55-65% humidity and 12-h light/12-h dark cycle. They were allowed free access to pelletized growers mash and clean drinking water. Prior to commencement of the study, the rats were acclimatized to the laboratory environment for one week. Standard experimental protocol was followed for this study.
Experimental Design
The rats were divided into 7 groups of 5 rats each: normal control, CCl4 control, extract control, silymarin, and 200, 300 and 400 mg/kg bwt extract groups. Cardiac injury was induced with CCl4 (1.0 mL/kg bwt orally, thrice weekly for two weeks) [16]. Rats in the extract control were not exposed to the toxicant, but received 400 mg extract/kg bwt throughout the period of treatment. Silymarin group rats were administered 100 mg silymarin/kg bwt, while those in the three treatment groups received varied doses of the extract (200, 300 and 400 mg/kg bwt, respectively). Treatment lasted 14 days.
Blood and Tissue Sample Collection
At the end of the treatment period, the rats were euthanized and blood samples were collected via cardiac puncture in heparinized sample bottles. The hearts of all experimental rats were harvested, washed in ice-cold saline, blotted dry and placed in plain containers. Weighted portions of the organ were used to prepare 20 % tissue homogenate. The blood and tissue homogenate were subsequently centrifuged at 2000 rpm for 10 min to obtain plasma and supernatant, which were used for biochemical analysis.
Biochemical Analysis
The activities of AST, catalase and SOD were measured [17-19]. Atherogenic index of plasma (AIP), LDL-C, AC, and CRR were determined [20-22]. The levels of TP and MDA were also measured [23,24]. Glucose 6-phosphate dehydrogenase (G6PDH) activity was measured as the rate of conversion of NADP+ to NADPH with time in minutes.
Statistical Analysis
Data are expressed as mean ± SEM (n = 5). Statistical analysis was performed using SPSS (version 20). Groups were compared using Duncan multiple range test. Statistical significance was assumed at p < 0.05.
RESULTS
Effect of Aqueous Extract of I. trichanta Leaves on Weight Parameters
Induction of cardiac injury with CCl4 significantly reduced the body weight gained, but it increased the weight of the heart and relative organ weight (p < 0.05). However, treatment of the rats with aqueous extract of I. trichanta leaves led to significant improvement in the weight gained, while reducing the heart weight and relative organ weight (p < 0.05). The effect of the extract on relative organ weight was dose-dependent (Table 1).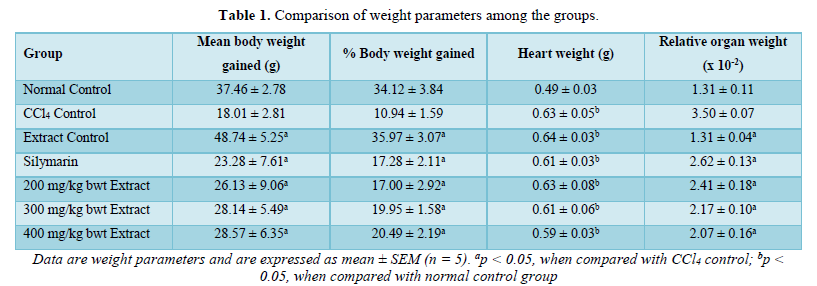
Effect of Aqueous Extract of I. trichanta Leaves on Cardiac Function and Lipid Peroxidation Index
As shown in Figures 1 & 2, CCl4 markedly elevated the levels of TP, MDA, LDL-C as well as activity of AST, but these parameters were significantly reduced by extract treatment (p < 0.05). The effect of the extract on LDL-C level followed a dose-dependent pattern.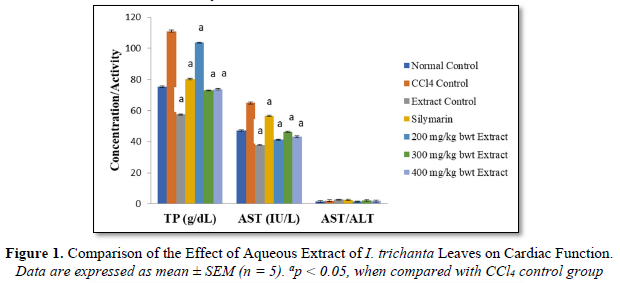
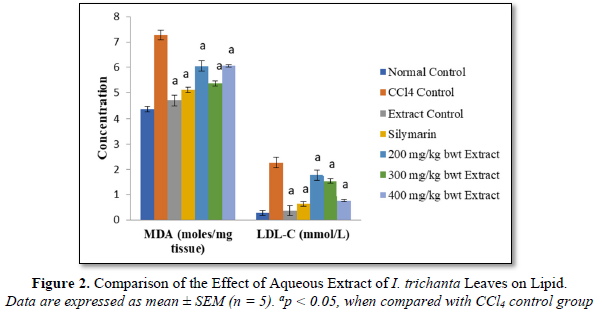
Effect of Aqueous Extract of I. trichanta Leaves on Cardiac Oxidative Status
The activities of SOD, catalase and G6PDH were significantly reduced by CCl4 intoxication, but they were increased after treatment with aqueous extract of the medicinal plant (p < 0.05) (Figure 3). The extract promoted the activities of SOD and catalase in a dose-dependent manner.
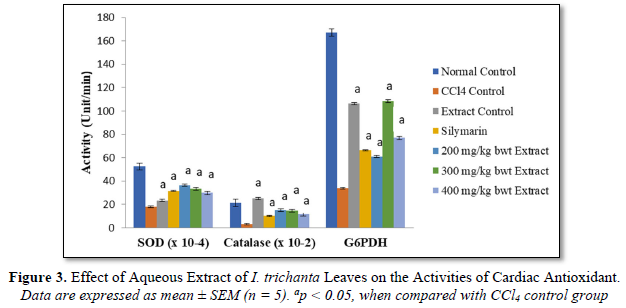
Effect of Aqueous Extract of I. trichanta Leaves on Cardiovascular Disease Factors
Atherogenic index of plasma (AIP), AC and CRR were markedly elevated by the cardiotoxic agent CCl4, but these parameters were reduced significantly and dose-dependently after treatment with the extract (p < 0.05). These results are shown in Figure 4.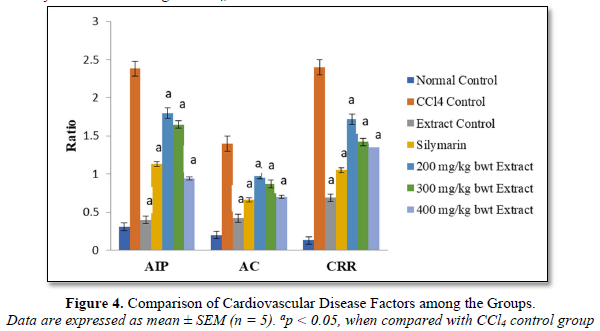
DISCUSSION
Cardiotoxicity induced by drugs poses a serious risk to human health [25]. Carbon tetrachloride (CCl4) is highly toxic to organs/tissues such as liver, kidneys, heart, lung, testis, brain and blood [26]. Once absorbed, it is widely distributed among tissues, especially those with high lipid content. The compound is biotransformed by hepatic microsomal cytochrome P450 (CYP2E1) to trichloromethyl radical, which initiates lipid peroxidation [27,28]. Free radicals formation, a rate limiting process in tissue peroxidative damage, is the generally accepted mechanism of CCl4-induced cardiotoxicity [29].
Reactive oxygen species (ROS) and oxidative stress have been shown to play an important role in the etiopathogenesis of tissue injury. The role of oxidative stress in cardiac hypertrophy and remodeling has been demonstrated. In atherogenesis, increased generation of ROS in the vascular wall and a reduction in the bioavailability of nitric oxide (NO) lead to endothelial dysfunction [30,31].
Bioactive agents from natural sources have gained wide acceptability in modern medicine, as they are known to reduce the risk of cardiac ailments by either scavenging free radicals or halting their formation [32]. Most of the pharmacologically important drugs are derived from plants. Plant derivatives used as drugs play crucial role in health-care systems around the globe. They are not only used for the management of disease conditions but also for maintenance of proper health [33]. The aim of this study was to investigate the cardioprotective capacity of aqueous extract of Icacina trichanta leaves in rats exposed to CCl4.
Aspartate aminotransferase (AST) is a marker of cardiac function and an increase in its activity reflects the functional state of the heart. Damage to the cardiomyocytes membrane (increased permeability of the plasma membrane) results in the leakage of this enzyme into systemic circulation. Although hepatocytes also express AST, the use of AST/ALT ratio gives an indication of the source of the enzyme. Increased total protein level is seen in some disease conditions [34]. In this study, CCl4 markedly elevated the levels of total protein, MDA, LDL-C as well as activity of AST, but these parameters were significantly reduced by extract treatment. An elevated LDL-C level is associated with increased risk for heart disease and stroke. Low-density lipoprotein (LDL) transports cholesterol to the arteries, and when its level is elevated, this cholesterol accumulates in blood vessel walls and contributes to the formation of plaque [35].
Superoxide dismutase (SOD) detoxifies superoxide anion (O2•-) which otherwise damage cell membrane and macromolecules [36]. Catalase plays an important role in antioxidant defense system. In animals, hydrogen peroxide is detoxified by catalase and glutathione peroxidase (GPx). Catalase protects cells from hydrogen peroxide generated within them [37].
Malondialdehyde (MDA), a commonly used biomarker of lipid peroxidation, is a breakdown product of lipid peroxyl radicals. Measured level of MDA is considered a direct index of oxidative injuries associated with lipid peroxidation [38].
In this study, G6PDH assay was employed as an indirect way of assessing the level of glutathione (reduced), since the reaction catalyzed by G6PDH in the erythrocyte membrane and cells of other sensitive tissues provides the coenzyme, NADPH which furnishes the hydride ion (or hydrogen) needed to keep or maintain glutathione in the reduced state, where it is active as a free radical scavenger. The results obtained in this study showed that the activities of SOD, catalase and G6PDH reduced by CCl4 intoxication, were significantly increased after treatment with aqueous extract of the medicinal plant. The extract promoted the activities of SOD and catalase in a dose-dependent manner. Treatment with aqueous extract of I. trichanta leaves significantly decreased all the cardiovascular disease risk factors, measured in this study. The effects were comparable to those of the standard cardioprotective drug, silymarin. The protective property of the extract may be attributed to its phytochemicals which act as antioxidants [10,16]. Plants rich in phenolics are reported to function as good antioxidants [39-41]. Similarly, plants containing important phytochemicals have been shown to confer protection on vital organs in animals [42-47].
CONCLUSION
The results of this study have shown that I. trichanta is cardioprotective: aqueous extract of the plant leaves significantly reversed CCl4-induced cardiac injury in rats, and it compares favorably with silymarin. The effects of CCl4 on rat heart were ameliorated on administration of graded doses of the extract.
- Colombo A, Meroni CA, Cipolla CM, Cardinale D (2013) Managing cardiotoxicity of chemotherapy. Curr Treat Options Cardiovasc Med 15(4): 410-424.
- Benson JM, Tibbetts BM, Thrall KD, Springer DL (2001) Uptake, tissue distribution, and fate of inhaled carbon tetrachloride: Comparison of rat, mouse, and hamster. Inhal Toxicol 13: 207-217.
- Iqubal A, Iqubal MK, Sharma S, Ansari MA, Najmi AK, et al. (2019) Molecular mechanism involved in cyclophosphamide-induced cardiotoxicity: Old drug with a new vision. Life Sci 218: 112-131.
- Shanmugam G, Challa AK, Litovsky SH, Devarajan A, Wang D, et al. (2019) Enhanced Keap1-Nrf2 signaling protects the myocardium from isoproterenol-induced pathological remodeling in mice. Redox Biol 27: 101212.
- Prince PSM, Dhanasekar K, Rajakumar S (2015) Vanillic acid prevents altered ion pumps, ions, inhibits Fas-receptor and caspase mediated apoptosis-signaling pathway and cardiomyocytes death in myocardial infarcted rats. Chem Biol Interact 232: 68-76.
- Lin JK, Moran AE, Bibbins-Domingo K, Falase B, Tobias AP, et al. (2019) Cost-effectiveness of a fixed-dose combination pill for secondary prevention of cardiovascular disease in China, India, Mexico, Nigeria, and South Africa: A modeling study. Lancet Glob Health 7(10): e1346-1358.
- Hemalatha KL, Prince PSM (2016) Anti-inflammatory and antithrombotic effects of zingerone in a rat model of myocardial infarction. Eur J Pharmacol 791: 595-602.
- Brattin WJ, Glende EA, Recknagel RO (1985) Pathological mechanisms in carbon tetracholoride hepatotoxicity. J Free Radic Biol Med1(1): 27-38.
- Grabley T, Akuodor CW (2010) Medicinal Plants and Traditional Medicine. Watermelon Crop Information. Cucurbit Breeding Horticultural Science.
- Abu OD, Onoagbe IO (2019) Biochemical effect of aqueous extract of Dialium Guineense stem bark on oxidative status of normal Wistar rats. Int J Clin Biol Biochem 1(2): 15-18.
- Sofowora A (1993) Medicinal Plants and Traditional Medicine in Africa. 2nd Spectrum Books Ltd., Ibadan, Nigeria. pp: 289.
- Akah PA, Nwabie AI (1994) Evaluation of Nigerian traditional medicinal plants used for rheumatic inflammatory disorders. J Ethnopharmarcol 42: 179-182.
- Iwu MM (1983) Traditional Igbo Medicine, Report of a project sponsored by the Institute of African Studies, University of Nigeria, Nsukka. pp: 48.
- Timothty O, Idu M, (2011) Preliminary phytochemistry and in vitro antimicrobial properties of aqueous and methanol extracts of Icacina trichantha Leaf. Int J Med Arom Plants 1: 1-5.
- Abu OD, Imafidon KE, Iribhogbe ME (2015) Biochemical effect of aqueous leaf extracts of Icacina trichanta Oliv on urea, creatinine and kidney oxidative status in CCl4-induced Wistar rats. Niger J Life Sci 5(1): 85-89.
- Abu OD, Imafidon KE, Iribhogbe ME (2017) Aqueous leaf extract of Icacina trichanta Oliv Ameliorates CCl4-induced liver toxicity in Wister rats. J Niger Soc Exper Biol 17(3): 107-111.
- Reitman S, Frankel SA (1957) Colorimetric method for the determination of serum glulamic oxaloacetic and glulamic pyranc transaminases. Am J Chin Pathol 28: 56-59.
- Cohen G, Dembie CD, Marcus J (1970) Measurement of catalase activity in tissue extracts. Anal Biochem 34: 30-38.
- Misra HR, Fridovich I (1972) The role of superoxide anions in the auto oxidation of epinephrine and a single assay for superoxide dismutase. J Biol Chem 247: 3170-3175.
- Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18(6): 499-502.
- Ikewuchi CJ, Ikewuchi CC (2009) Alteration of Plasma Lipid Profiles and Atherogenic Indices by Stachytarpheta jamaicensis L. (Vahl). Biokemistri 21(2): 71-77.
- Frohlich J, Dobiášová M (2003) Fractional Esterification Rate of Cholesterol and Ratio of Triglycerides to HDL-Cholesterol are Powerful Predictors of Positive Findings on Coronary Angiography. Clin Chem 49(11): 1873-1880.
- Henry RJ, Sobel C, Beckman S (1957) Determination of serum protein by the Biuret reaction. Anal Chem 92(149): 1-5.
- Guttridge JMC, Wilkins C (1982) Cancer dependent hydroxyl radical damage to ascorbic acid. Formation of thiobarbituric acid reactive product. FEBS Lett 137: 327-340.
- Dessalvi CC, Deidda M, Mele D, Bassareo P, Esposito R, et al. (2018) Chemotherapy-induced cardiotoxicity: New insights into mechanisms, monitoring, and prevention. J Cardiovasc Med 19(7): 315-323.
- Adaramoye OA (2009) Comparative effects of vitamin E and kolaviron (a biflavonoid from Garcinia kola) on carbon tetrachloride induced renal oxidative damage in mice. Pak J Biol Sci 12: 1146-1151.
- Shenoy KA, Somayaji SN, Bairy KL (2001) Hepatoprotective effects of Ginkgo biloba against carbon tetrachloride induced hepatic injury in rats. Indian J Pharmacol 33: 260-266.
- Adewole S, Salako A, Doherty O, Naicker T (2010) Effect of melatonin on carbon tetrachloride-induced kidney injury in Wistar rats. Afr J Biomed Res 10: 34-41.
- Plaa GL, Witschi H (1976) Chemicals, drugs, and lipid peroxidation. Ann Rev Pharmacol Toxicol 16: 125-142.
- Lee R, Margaritis M, Channon KM, Antoniades C (2012) Evaluating oxidative stress in human cardiovascular disease: Methodological aspects and considerations. Curr Med Chem 19(16): 2504-2520.
- Channon KM, Guzik TJ (2002) Mechanisms of superoxide production in human blood vessels: relationship to endothelial dysfunction, clinical and genetic risk factors. J Physiol Pharmacol 53: 515-524.
- Wang CZ, Mehendale SR, Yuan CS (2007) Commonly used antioxidant botanicals: active constituents and their potential role in cardiovascular illness. Am J Chin Med 35(04): 543-558.
- Dec GW (2003) Digoxin remains useful in the management of chronic heart failure. Med Clin 87(2): 317-337.
- Tietz NW (1995) Clinical Guide to Laboratory Tests. 3rd WB Saunder’s Company, Philadelphia. pp: 518-519.
- Reiser R, Probstfield JL, Silvers A, Scott LW, Shorney ML, et al. (1985) Plasma lipid and lipoprotein response of humans to beef fat, coconut oil and safflower oil. Am J Clin Nutr 42(2): 190-197.
- Teixeira HD, Schumacher RI, Meneghini R (1998) Lower intracellular hydrogen peroxide levels in cells overexpressing CuZn-superoxide dismutase. Proc Natl Acad Sci 95: 7872-7875.
- Speranza MJ, Bagley AC, Lynch RE (1993) Cells enriched for catalase is sensitized to the toxicities of bleomycin, adriamycin, and paraquat. J Biol Chem 268: 19039-19043.
- Khan RA, Khan MR, Sahreen S, Bokhari J (2010) Prevention of CCl4- induced nephrotoxicity with Sonchus asper in rat. Food Chem Toxicol 48(8): 2469-2476.
- Abu OD, Onoagbe IO, Obahiagbon O (2020a) In Vitro Antioxidant Activities of Extracts of Dialium Guineense Stem Bark. Am J Sci Eng Res 3(4): 68-75.
- Abu OD, Onoagbe IO, Obahiagbon O (2020b) Phenolic contents of extracts of Dialium guineense stem bark. Am J Sci Eng Res 3(4): 92-96.
- Abu OD, Onoagbe IO, Obahiagbon O (2020c) Analyses of metal and amino acid compositions of aqueous and ethanol stem bark extracts of Dialium guineense. J Biogene Sci Res 6(4): 1-3.
- Abu OD, Umar A-B, Ajuwa OI (2022) Protective Property of Total Saponins and Tannins of Dialium guineense Stem Bark in CCl4-Induced Cardiotoxicity in Rats. World J Gene Mol Biol 1: 1-6.
- Abu OD, Ezike TV, Ajuwa OI (2022) Cardioprotective property of extracts of Dialium guineense stem bark in rats exposed to CCl4. Am J Biomed Sci Res 2022: 689-693.
- Abu OD, Iyare HE, Ogboi KU (2022) Antioxidant Property of Total Saponins and Tannins of Dialium guineense Stem Bark in Rats Hearts Exposed to CCl4. J Clin Epidemiol Toxicol 3(3): 1-4.
- Abu OD, Omage JI, Ogbebor EO (2022) Effect of Total Saponins and Tannins Isolated from the Stem Bark of Dialium guineense on Lipid Profile and CCl4- Induced Histological Changes in Liver of Wistar Rats. J Med Biol 3(2): 1-9.
- Abu OD, Orobator ON, Momodu IB (2022a) Evaluation of the Effect of Total Saponins and Tannins Isolated from Dialium guineense Stem Bark on CCl4 - Induced Hepatotoxicity in Wistar Rats. Glob J Med Clin Case Rep 9(3): 035-038.
- Abu OD, Orobator ON, Momodu IB (2022b) Investigation of the Hepatoprotective Effect of Extracts of Dialium guineense Stem Bark in Wistar Rats Exposed to CCl J Clin Gastroenterol Hepatol 4(2): 123-126.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Agriculture and Forest Meteorology Research (ISSN:2642-0449)
- Proteomics and Bioinformatics (ISSN:2641-7561)
- Journal of Genomic Medicine and Pharmacogenomics (ISSN:2474-4670)
- Journal of Womens Health and Safety Research (ISSN:2577-1388)
- Journal of Microbiology and Microbial Infections (ISSN: 2689-7660)
- Journal of Biochemistry and Molecular Medicine (ISSN:2641-6948)
- Food and Nutrition-Current Research (ISSN:2638-1095)






