Case Report
Insertion-Site Necrosis of the Single-Rod Subdermal Contraceptive Implant: Case Reports
5241
Views & Citations4241
Likes & Shares
Implant-site necrosis is a rare complication. Local pain was the main symptom, appearing within 35 days of placement. Ambulatory multidisciplinary treatment was undertaken. Local debridement and implant removal was performed in 4 out of five patients. Time to complete healing varied from 45 days -12 months, depending on the wound.
Keywords: Contraceptive implant, Insertion-Site necrosis, Complication, Severe side effects
INTRODUCTION
The single-rod subdermal contraceptive implant containing etonogestrel (Implanon® MSD Merck Sharp & Dohme; Cazadores de Coquimbo 2841, Buenos Aires, Argentina), approved by the Food and Drug Administration in 2006, offers 3-year-long, reversible contraception [1], is over 99% effective, with a Pearl rate between 0.00-0.14 [2], and discontinuity rate of 16% per year of use [3]. Implant insertion-site complications are rare, with an incidence ranging from 0.3%-3.6% [4,5]. Implant insertion-site necrosis is a rare adverse effect, with scarce literature published worldwide. It comprises variable levels of tissue damage, ranging from local whitening, edema and reddening, to subcutaneous cellular tissue damage. The physiopathology is not entirely clear but could be generated by barium sulphate allergy, insertion-site infection, or vascular damage due to embolism, vasospasm, or inflammation secondary to a local cytotoxic drug [5-11].
OBJECTIVE
To present clinical manifestations and treatment of 5 cases of subdermal contraceptive implant insertion-site necrosis.
MATERIALS AND METHODS
Medical records’ information of women in whom Implanon® was placed were reviewed in search of insertion-site complications occurring between February 2015 and September 2019. Written consent was obtained from all patients on whom the report is based. Sexual and Reproductive Health National Program.
CLINICAL CASES
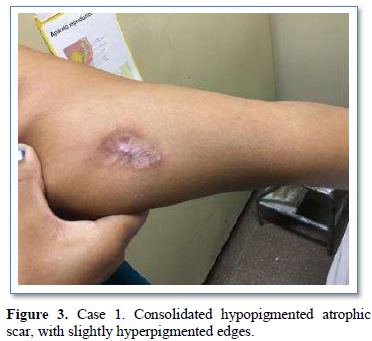
Of the 995 implanted patients, five presented insertion-site necrosis (0.5%). It represented 16% of implant-related early discontinuation causes. The events were reported to the manufacturer, and to the Sexual and Reproductive Health National Program in Argentina, that reports to the ANMAT. Clinical presentation and treatment details are summarized in Table 1. Symptom onset was within 35 days of implant placement, with patients referring pain as the main symptom. An ambulatory multidisciplinary approach to patient treatment was undertaken, in cooperation with the Plastic Surgery and Infectology Departments. None showed compromise beyond the subcutaneous cellular tissue. No abscesses were found and none progressed towards gangrene or sepsis. Local debridement was performed in four patients, one of them requiring necrotic tissue removal in an operating room, under locoregional plexus nerve block. In these 4 patients, the implant was also removed. All but one received antibiotic treatment, even in the absence of pathogens in tissue cultures. Tissue biopsy indicated a severe inflammatory process, with epidermal ulcer and areas of necrosis. Time until complete healing varied from 45 days-12 months, depending on the extent of the wound.
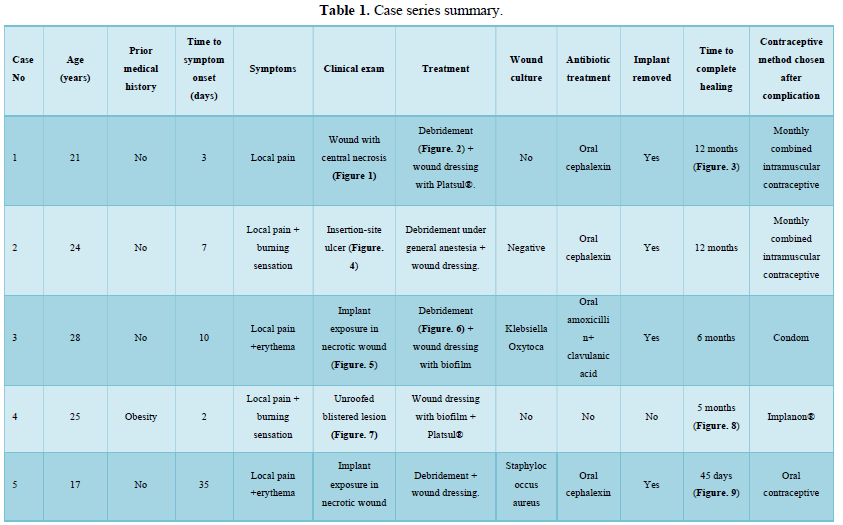
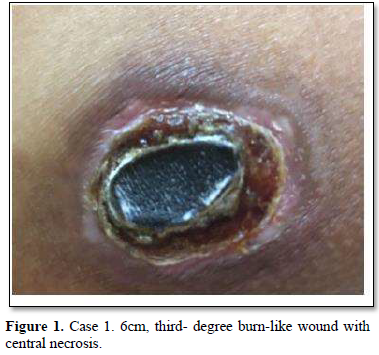
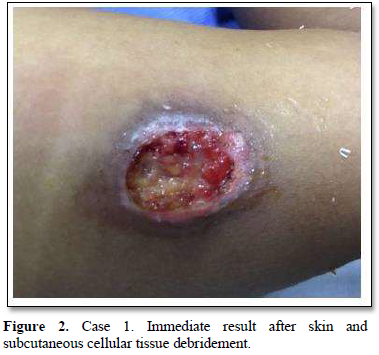
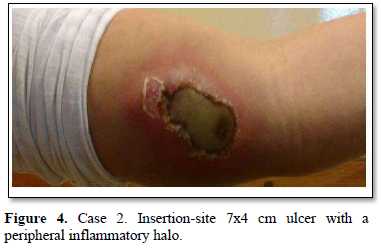
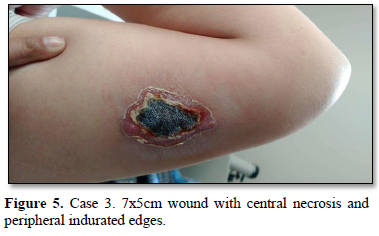
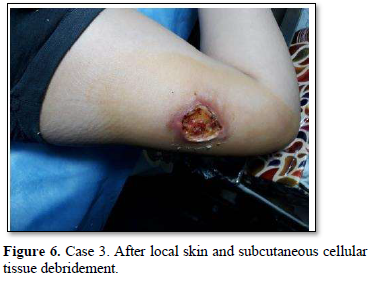
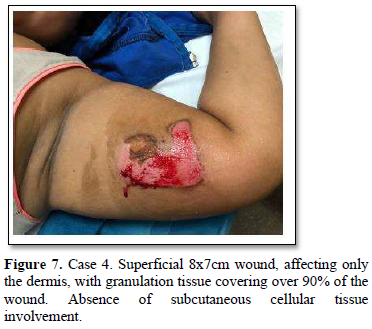
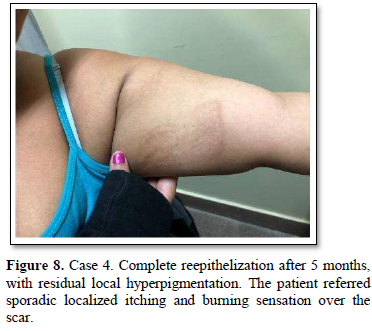
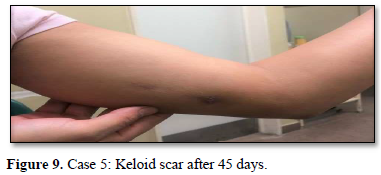










COMMENT
The information presented in this report does not aim to question the safety of Implanon®, but to describe a potential implant-related severe side effect. A published U.S. experience did not report cases of skin necrosis following these insertions [12]. Those that occurred in our cohort were not associated with a particular inserting physician or local anesthesia used. The implants in this series were placed by trained physicians, with a standardized technique. Insertion-site erythema or pain should alarm about the development of local complications. In the presence of necrotic tissue, wound debridement is the treatment of choice, and in most cases, the implant should be removed. Early diagnosis and multidisciplinary treatment are essential to avoid major life-threatening complications, severe aesthetic and/or functional damages.
ACKNOWLEDGMENTS
To the chiefs and staff of the Gynecology and Obstetrics Departments to the Staff of the Sexual and Reproductive Health Program, who provide us the supplies to our work.
To Dr. C. Chwat that assisted in the drafting of this study.
CONCLUSION
The subdermal implant is an excellent tool to prevent and reduce teenage pregnancy. Although the incidence of necrosis after its placement is low, we consider important to know this side effect, its form of presentation in order to make an acute diagnosis and treatment.
- Peterson HB, Kathryn MC (2005) Clinical practice: Long-acting methods of contraception. N Engl J Med 353: 2169-2175.
- Graesslin O, Tjeerd K (2008) The contraceptive efficacy of Implanon: A review of clinical trials and marketing experiences. Eur J Contracept Reprod Health Care 13: 4-12.
- James T (2008) Reducing unintended pregnancy in the United States. Contraception 77: 1-5.
- Ramdhan RC, Simonds E, Wilson C, Loukas M, Oskouian RJ, et al. (2018) Complications of Subcutaneous Contraception: A Review. Cureus 10(1): e2132.
- Pedroso C, Martins I, Palma F, Machado AI (2015) Implant site Nexplanon reaction? BMJ Case Rep: bcr2014206256.
- Serati M, Bogani G, Kumar S, Cromi A, Ghezzi F (2015) Delayed-type hypersensitivity reaction against Nexplanon. Contraception 91(1): 91-92.
- Niederhauser A, Magann EF, Hoffman K (2011) An allergic reaction to Implanon placement and review of the literature. J Ark Med Soc 108: 92.
- Sullivan MJ (2012) Allergy to nexplanon. J Fam Plann Reprod Health Care 38: 272.
- Partridge R, Bush J (2013) Infections post-Nexplanon® insertion. J Fam Plann Reprod Health Care 39: 309-310.
- Maneshi A (2017) Nicolau Syndrome. Arch Iran Med 20(1): 60-64.
- Chaudhry F (2013) Adverse reaction to Nexplanon. J Fam Plann Reprod Health Care 39(3): 231-232.
- Creinin M, Kaunitz A, Darney P, Lisa S, Tonja, et al. (2017) The US etonogestrel implant mandatory clinical training and active monitoring programs: 6-year experience. Contraception 95: 205-210.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Proteomics and Bioinformatics (ISSN:2641-7561)
- Journal of Genetics and Cell Biology (ISSN:2639-3360)
- Journal of Biochemistry and Molecular Medicine (ISSN:2641-6948)
- Journal of Veterinary and Marine Sciences (ISSN: 2689-7830)
- Food and Nutrition-Current Research (ISSN:2638-1095)
- Journal of Genomic Medicine and Pharmacogenomics (ISSN:2474-4670)
- Journal of Agriculture and Forest Meteorology Research (ISSN:2642-0449)











