2432
Views & Citations1432
Likes & Shares
Results: The statistical analysis showed that the treatment group, who received a dose of mifepristone, got their menses significantly earlier than the control cohort (mean No of Days; 3.54±1.60 vs 4.53±1.44) (p-value= <0.001).
Conclusion: This study showed that the use of mifepristone, along with other steroidogenic suppression agents, significantly reduces the number of days till menses, therefore reducing the hospital stay. This is fundamentally critical, as the safe reduction of hospital stay will benefit the oocyte donors both economically and socially.
Keywords: Oocyte donors, Menses induction, OHSS, IVF/ICSI, Patient care/safety
The Indian Council of Medical Research (ICMR) does not permit oocyte donation with a known donor who maybe a family, friend and/or relative of the couple. Hence seeking commercially motivated oocyte donors is the only legal modality available for these couples. Oocyte donors especially in India belong to low socio-economic strata [9]. Hence, donating their oocytes helps them earn substantially more than what they would otherwise. However, oocyte donation is associated with significant medical risks to the donors [9,10]. These risks are poorly addressed by many ART clinics. Some of these side effects include pain, infection, bleeding, ovarian torsion, ovarian hyper-stimulation syndrome (OHSS) [10,11]. OHSS is the most serious iatrogenic complication associated with in-vitro fertilization treatment. There is a universal practice of giving oocyte donors a GnRH agonist trigger, but despite this, there is a risk of OHSS. 5% of all patients undergoing controlled ovarian stimulation (COS) suffer from OHSS [11]. In most of these cases OHSS is mild which leads to temporary discomfort, however, severe cases, though rare, can be life-threatening causing massive ascites, marked ovarian enlargement, pleural effusion, hypovolemia with hypotension and oliguria, and electrolyte imbalance [12]. OHSS is significantly increased in oocyte donors as majority of them are
Mifepristone (RU-486) is a drug that is historically used in medical practice as an abortifacient and a contraceptive [14]. The mechanisms of action of RU-486 are to competitively act as an antagonist to progesterone and glucocorticoid receptors in the endometrium. Its anti-progestin property causes decidual necrosis by primarily blocking the endometrial progesterone receptors [14,15]. Furthermore, it is also shown to impede the production of human chorionic gonadotropin (hCG) in the placenta [15,16]. Mifepristone given to women undergoing COS post OPU as a therapeutic agent causes inhibition of progesterone during the luteal phase of the menstrual cycle leading to menses [17]. The onset of menses resets progesterone and estrogen levels, hence decreasing the risk of OHSS. Oocyte donors experience symptoms like pain, distention of abdomen and pelvic discomfort because of COS and OPU [17,18]. It is important for oocyte donors to get back to their normal life as soon as possible in a stable condition after their OPU, as many of these women are the primary bread winners of their family. The women coming for oocyte donation are selected based on their age (23 years to 35 years old) and parity (at least 1 living issue), hence majority of these women have young children who would require maternal care [19].
As per our hospital policy, oocyte donors are admitted post OPU till the onset of menses, to ensure their safety and circumvent complications associated with ovarian torsion and severe OHSS. The aim of this study was to investigate if administration of mifepristone would induce an early period for oocyte donors post OPU to enable them to return to their normal routine.
MATERIALS AND METHODS
Study Population
Oocyte donors who underwent OPU at Gunasheela Surgical & Maternity Hospital from January 2015 - December 2020 were recruited in this study. The study design was retrospective case control in nature and was approved by xxxx institutional ethics committee (Gunasheela Institutional Ethics Committee). Patients were divided into control (no mifepristone group) and treatment group (Mifepristone group) based on the year of recruitment. Donors who underwent OPU between January 2015- December 2017 belonged to the control group (n=107) and the ones between January 2018-December 2020 belonged to the treatment group (n=150). The donors were recruited based on the following criteria; age 2, anti-müllerian hormone (AMH) levels >1.3ng/ml, antral follicle counts (AFC) ≥12 follicles in both ovaries combined, negative viral screening test. Donors were also selected based on their parity (at least 1 live child of their own with a minimum age of 3 years) and no significant familial genetic abnormalities/disorders.
Controlled ovarian stimulation (COS) and OPU
Donors were started on COS on day 3 of their menstrual cycle using the antagonist protocol with daily injections of recombinant human follicle stimulating hormone (r-hFSH-alfa [Gonal‐f®, Merck Healthcare KGaA, Darmstadt, Germany]). From day 6 of stimulation gonadotropin-releasing hormone (GnRH) antagonist was started [(Ganirelix 0.25mg SC) (Orgalutran, N.V. Organon, Netherlands) till the day of trigger. Once at least 3 follicles reached a size of ≥18mm on transvaginal ultrasound (TVS), GnRH agonist trigger (Leuprolide Acetate, [Luprofact™4 Zydus Cadila Healthcare Ltd, Ahmedabad, India) was given. 35 hours after the trigger, oocyte retrieval under TVS (Ultrasound Machine, E8C-RS 10Hz probe, General Electric Healthcare, Chicago, United States of America) guidance Oocyte aspiration was performed under general anesthesia with a 16 gauge single lumen needle (Cooks Medical®, Ovum Aspiration Single Lumen Needle) and follicles were aspirated from the ovaries with a suction pressure between 80 to 100 mmHg. Aspirated follicular fluid was handed over to the embryologist for screening of oocytes followed by vitrification or intra cytoplasmic sperm injection (ICSI).
Post OPU care of donors
After the OPU procedure, the donors were kept under observation. The patients were started on injection GnRH antagonist [(Inj. Antag 0.25mg) (Ganirelix 0.25mg SC) (Orgalutran, N.V. Organon, Netherlands),ogranon)], tablet cabergoline [(0.5mg O.D) (Cabercet, Synokem pharmaceuticals ltd, Uttarakhand, India)], tablet Letrozole IP [(2.5mg T.I.D) (Letpro, aristo pharma ltd, Maharashtra India)] from the day of OPU for 5 days. As a hospital protocol, donors were admitted in the hospital till the onset of menses. Donors admitted from January 2018-December 2020 were given tablet mifepristone (600mg stat) (Mifegest, Zydus Fortiza, Sikkim, India)] on post OPU day 3. However, donors admitted from January 2015- December 2017 were not given mifepristone.
Statistical Analysis
Post HOC power calculation to detect the difference of one day in the primary outcome was estimated to be 99%. Number and percentage were reported for all categorical data. Continuous data were summarized as mean and standard deviation. The Pearson Chi-square test and Fisher’s exact test (less cell count) were done for assessing the association between categorical variables. Student T-test was used to compare mean values with mean differences and 95% confidence intervals. All tests were two-sided at α=0.05 level of significance. All analyses were done using Statistical Package for Social Sciences (SPSS) software Version 21.0 (Armonk, NY: IBM Corp).
RESULTS
There was a total of 257 oocyte donors recruited for this study. Amongst these, 150 oocyte donors were grouped under the mifepristone cohort and 107 oocyte donors were grouped in the non-mifepristone cohort.
Table 1 describes the baseline characteristics and ovarian reserve markers for oocyte donors. It was observed that the mean age of oocyte donors in the mifepristone group is 24.4 ±9.2 years of age compared to the non-mifepristone group is 25.89 ± 6.4 (p-value =0.150). It was also noted that mean BMI in the mifepristone was 22.4 ± 4.5 kg/m2 and in the non-mifepristone group 23.1 ± 4.2 kg/m2 (p-value 0.078). The ovarian reserve markers between these groups were also comparable; AMH in the mifepristone group was 4.34 ±0.23 ng/ml and in the non-mifepristone group it was recorded as 4.13 ±0.67 ng/ml (p-value=0.227). AFC in both was also noted to be similar (n=20 ±3.4 vs. 19 ±3.2) (p-value 0.003). In the hormone parameters on the day of trigger the mean serum E2 levels between were also similar (2857± 812 vs 2750± 900 pg/ml) (p-value =0.009). Finally, the mean total oocytes that were aspirated from oocyte donors were also seen to be notably similar (n=15±3 vs. 14±3) (p-value= 0.001).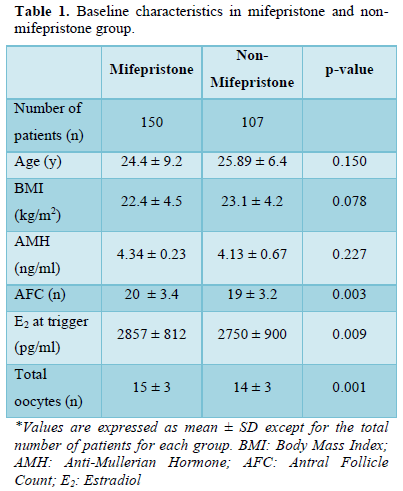
Figure 1 shows the difference in the number of days to get menses in oocyte donors between the treatment and the control cohort. It is evident that treatment group who received a dose of mifepristone got the menses significantly earlier than the control cohort (mean No of Days; 3.54±1.60 vs. 4.53±1.44) (p-value= <0.001).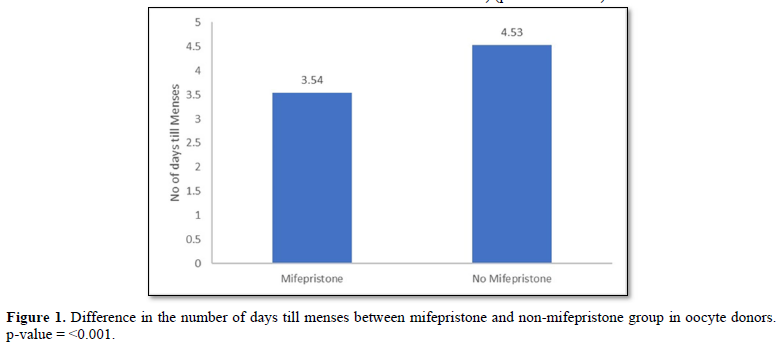
DISCUSSION
The primary goal for ART clinics who manage oocyte donors is to safely discharge them after their OPU procedure without the potential risk of OHSS. The results of this study indicated that patients who were given mifepristone, were significantly more likely to get their menses earlier than patients who did not receive mifepristone. Furthermore, the onset of menses is deemed to be an important milestone in patient recovery as this is directly correlated with reduced likelihood of OHSS [11,20]. In a normal COS cycle patients are expected to get their menses anywhere between 10 days to 2 weeks from the day of OPU [21]. Wang [21] reported that early OHSS is associated with high secretions of progesterone from the corpus luteum and the rapid decrease in this hormone through menstruation will alleviate the risk of potential OHSS. Mifepristone is a progesterone receptor antagonist which promotes premature menstruation, therefore abating the risk of OHSS [17,18,22]. Literatures suggest that many protocols of steroidogenic suppression after OPU (in the luteal phase) rely on cocktail regimen to reduce OHSS. Wang [21] and Luo [22] suggested the use of the GnRH Antagonist (Inj Antag, 0.25mg, SC x5 days from day 1 post OPU), aromatase inhibitor (Letrozole 2.5mg BD x5 days from day 1 post OPU) and mifepristone (Tablet 25mg BD, x3, from day 1 post OPU) together is shown to be most effective in reducing OHSS. Luo [22] further showed the reduction of OHSS by induction of menses by comparing these drugs individually and as a cocktail regimen. Their results stated that mifepristone alone will induce menses faster (10.5±1.9 days) compared to the GnRH antagonist group (10.7±2.4 days) and letrozole (10.9±2.6 days). The durations of induction of menses were the same between the mifepristone and the cocktail regimens group (10.4± 2.3days). In our study we decided to maintain the cocktail therapy for oocyte donors using GnRH antagonist, letrozole and cabergoline in the non-mifepristone cohort. However, in the treatment group Mifepristone was added with the other cocktail therapy drugs. Cabergoline is an ergot derivative and potent dopamine receptor agonist. It thus prevents the phosphorylation of VEGF-receptor 2, therefore reducing the in vitro and in vivo release of vasoactive angiogenic agents. This consequently reduces vascular permeability [24,25]. Cabergoline is given as a preventive strategy to reduce the severity of OHSS [23,24].
Many studies argue that the administration of GnRH agonist trigger during in a GnRH Antagonist cycle is sufficient to fully evade the risk the of OHSS [23,26,27]. However, there are studies that suggest that OHSS can still be invoked upon GnRH agonist trigger [28]. Furthermore, there is a lot of speculation that GnRH, FSH, or LH receptor gene mutations can lead to OHSS predisposition [28,29]. Additional research is needed in this field to fully understand the mechanisms of this phenomenon. Clinicians should be vigilant to the signs and symptoms of OHSS post OPU, and additionally other therapeutic drugs should be considered for its prophylaxis.
The use of mifepristone to prevent OHSS has been poorly studied in previous literatures. We have shown that the use of this drug in a large-scale Indian population will significantly reduce the hospital stay of oocyte donors in our center by the induction of early menses. A small pilot study conducted in our center involving 20 oocyte donors showed that addition of mifepristone for steroidogenic suppression to manage OHSS resulted in a significant reduction in the duration of hospital stay. Unfortunately, due to funding constrains this data was not published. Hence, after 2017, mifepristone was added to the steroidogenic suppression protocol in our hospital to manage OHSS in oocyte donors.
Our study is first of its kind to shed light on the social issues related to oocyte donors in India. According to National Assisted Reproductive Technology Registry of India [30,31], oocyte donation in India has exponentially increased from 2007 to 2009 (1047-2130). However, there are no official statistics available that state the number of the oocyte donation cycles carried out in India currently. Additionally, this increase can be associated with the Indian society becoming more liberal in accepting donor gametes and a larger population of young women going into premature ovarian insufficiency [32]. On the contrary, there are not any specific safety protocols for oocyte donors in ART clinics in India. In many of the ART clinics oocyte donors have been treated as day care patients with minimal to no requirement for hospital admission [33]. This is associated with increased likelihood of early OHSS, which if untreated will result in worsening of symptoms of OHSS and death in some cases [34]. On the other hand, oocyte donors in some clinics are admitted to hospital for prolonged duration, with no justified reason. This in turn will cause financial and emotional burden on the donors, as they are away from their family and place of work [9]. Therefore, mitigating the medical risks, while maintaining the decreased time in the hospital is crucial to ensure the wellbeing of oocyte donors.
STRENGTHS
This study is first of its kind population that evaluates the efficacy of mifepristone in promoting early menses in oocyte donors after OPU to enable early return to routine life and reduce the risk of OHSS in an Indian population. This also highlights the social aspects of oocyte donors in India, which in turn will invoke policy reforms to ensure safety (both physical and mental) for them. Another key strength of this study is that it analyses data from a large sample size from a single center over many years with power of the study being 99%.
LIMITATIONS
Due to the retrospective and observational nature of this study in a population setting it was difficult to decipher the pharmacodynamics and pharmacokinetic potential of mifepristone to induce early menses in women undergoing COS. In this study we have only considered the oocyte donor population. We need to study the use of mifepristone to induce early menses in the general infertile population at risk for OHSS (PCOS and previous hyper responders). A large-scale clinical trial would be beneficial to get concrete results using mifepristone as an adjuvant to reduce the risk of OHSS in women undergoing COS. Another limitation of this study would be that we did not consider targeted questionnaires for oocyte donors to get a scope of social and economic background to understand the need for early and safe discharge from hospital after OPU.
CONCLUSION
The risk of OHSS in oocyte donors is apparent after COS and OPU. Medical prevention of OHSS starts from the day of ovulation trigger till the day of discharge. We investigated whether the use of mifepristone along with other steroidogenic suppression will aid in invoking early menses, therefore reducing the duration of hospital stay of the oocyte donors. We were able to show that the use of mifepristone significantly reduces the number of days till menses, therefore reducing the hospital stay. This is fundamentally critical, as the safe reduction of hospital stay will benefit the oocyte donors both economically and socially.
DECLARATIONS
Conflict of interest
There are no conflicts of interest.
Acknowledgements
We thank and acknowledge all the efforts of the embryology department at the Gunasheela Surgical and Maternity Hospital for helping us collect the data.
Ethics approval
This study was approved by the Gunasheela Hospital Ethics Committee. Sanction number: EC/OA/30/2021.
Author Contributions
M.D, L.C.A, A.N and D.G were responsible for the conception and design of this. R.K was responsible for the analysis of data. All authors read and approved the final article.
Author Disclosure Statement
No competing financial interests exist.
Availability of Data and Materials
All data pertaining to this study are contained and presented in this article.
Funding Information
No funding was received for this article.
- Walker MH, Tobler KJ (2021) Female Infertility. StatPearls.
- Wolf DP, Byrd W, Dandekar P, Quigley MM (1984) Sperm concentration and the fertilization of human eggs in vitro. Biol Reprod 31(4): 837-848.
- Jadva V, Lamba N, Kadam K, Golombok S (2015) Indian egg donors' characteristics, motivations and feelings towards the recipient and resultant child. Reprod Biomed Soc Online 1(2): 98-103.
- Swain JE, Pool TB (2008) ART failure: Oocyte contributions to unsuccessful fertilization. Hum Reprod Update 14(5): 431-446.
- Hibino Y, Shimazono Y (2014) Impact of egg donation deliveries from domestic and overseas sources on maternal care: A questionnaire survey of Japanese perinatal physicians. Environ Health Prev Med 19(4): 271-278.
- Bayefsky MJ (2020) Legal and Ethical Analysis of Advertising for Elective Egg Freezing. J Law Med Ethics 48(4): 748-764.
- Masoumi SZ, Parsa P, Darvish N, Mokhtari S, Yavangi M, et al. (2015) An epidemiologic survey on the causes of infertility in patients referred to infertility center in Fatemieh Hospital in Hamadan. Iran J Reprod Med 13(8): 513.
- Tulay P, Atılan O (2019) Oocyte donors’ awareness on donation procedure and risks: A call for developing guidelines for health tourism in oocyte donation programmes. J Turkish Ger Gynecol Assoc 20(4): 236.
- Jadva V, Lamba N, Kadam K, Golombok S (2015) Indian egg donors’ characteristics, motivations and feelings towards the recipient and resultant child. Reprod Biomed Soc Online 1(2): 98.
- Jahromi BN, Parsanezhad ME, Shomali Z, Bakhshai P, Alborzi M, et al. (2018) Ovarian Hyperstimulation Syndrome: A Narrative Review of Its Pathophysiology, Risk Factors, Prevention, Classification, and Management. Iran J Med Sci 43(3): 248-260.
- Kumar P, Sait SF, Sharma A, Kumar M (2011) Ovarian hyperstimulation syndrome. J Hum Reprod Sci 4(2): 70.
- Jayaprakasan K, Herbert M, Moody E, Stewart JA, Murdoch AP (2007) Estimating the risks of ovarian hyperstimulation syndrome (OHSS): implications for egg donation for research. Hum Fertil (Camb) 10(3): 183-187.
- Maria B, Stampf F, Goepp A, Ulmann A (1988) Termination of early pregnancy by a single dose of mifepristone (RU 486), a progesterone antagonist. Eur J Obstet Gynecol Reprod Biol 28: 249-255.
- Cadepond F, Ulmann A, Baulieu EE (2003) RU486 (MIFEPRISTONE): Mechanisms of Action and Clinical Uses. Annu Rev Med 48: 129-156.
- Gemzell-Danielsson K, Marions L (2004) Mechanisms of action of mifepristone and levonorgestrel when used for emergency contraception. Hum Reprod Update 10(4): 341-348.
- Escudero EL, Boerrigter PJ, Bennink HJTC, Epifanio R, Horcajadas JA, et al. (2005) Mifepristone is an effective oral alternative for the prevention of premature luteinizing hormone surges and/or premature luteinization in women undergoing controlled ovarian hyperstimulation for in vitro J Clin Endocrinol Metab 90(4): 2081-2088.
- Formigli L, Badulli G, Rija R, Aimée M, Krishnamoorthy R, et al. (2019) Misoprostol (Cytotec) for prevention of ovarian hyperstimulation syndrome in cases of in vitro Clin Obstet Gynecol Reprod Med 5(1): 1-2.
- Gorrill MJ, Johnson LK, Patton PE, Burry KA (2001) Oocyte donor screening: the selection process and cost analysis. Fertil Steril 75(2): 400-404.
- Du D-F, Li M-F, Li X-L (2019) Ovarian hyperstimulation syndrome: A clinical retrospective study on 565 inpatients. Gynecol Endocrinol 36(4): 313-317.
- Shen X, Long H, Guo W, Xie Y, Gao H, et al. (2020) The ovulation trigger-OPU time interval of different ovarian protocols in ART: A retrospective study. Arch Gynecol Obstet 302(2): 519.
- Wang YQ, Luo J, Xu WM, Xie QZ, Yan WJ, et al. (2015) Can steroidal ovarian suppression during the luteal phase after oocyte retrieval reduce the risk of severe OHSS? J Ovarian Res 8: 63.
- Luo J, Qi Q, Chen Y, Wang Y, Xie Q (2021) Effect of GnRH-antagonist, mifepristone and letrozole on preventing ovarian hyperstimulation syndrome in rat model. Reprod Biomed Online 42(2): 291-300.
- Kılıç N, Özdemir Ö, Başar HC, Demircan F, Ekmez F, et al. (2015) Cabergoline for preventing ovarian hyperstimulation syndrome in women at risk undergoing in vitro fertilization/intracytoplasmic sperm injection treatment cycles: A randomized controlled study. Avicenna J Med 5(4): 123.
- Rains CP, Bryson HM, Fitton A (1995) Cabergoline. A review of its pharmacological properties and therapeutic potential in the treatment of hyperprolactinaemia and inhibition of lactation. Drugs 49(2): 255-279.
- Lainas GT, Kolibianakis EM, Sfontouris IA, Zorzovilis IZ, Petsas GK, et al. (2021) Outpatient management of severe early OHSS by administration of GnRH antagonist in the luteal phase: An observational cohort study. Reprod Biol Endocrinol 10: 69.
- Lee D, Kim SJ, Hong YH, Kim SK, Jee BC (2017) Gonadotropin releasing hormone antagonist administration for treatment of early type severe ovarian hyperstimulation syndrome: A case series. Obstet Gynecol Sci 60(5): 449.
- Xing W, Lin H, Li Y, Yang D, Wang W, et al. (2015) Is the GnRH Antagonist Protocol Effective at Preventing OHSS for Potentially High Responders Undergoing IVF/ICSI? PLoS One 10(10): e0140286.
- Stouffs K, Daelemans S, Santos-Ribeiro S, Seneca S, Gheldof A, et al. (2019) Rare genetic variants potentially involved in ovarian hyperstimulation syndrome. J Assist Reprod Genet 36: 491-497.
- Malhotra N, Shah D, Pai R, Pai HD, Bankar M (2013) Assisted reproductive technology in India: A 3 year retrospective data analysis. J Hum Reprod Sci 6(4): 235-240.
- National Registry of Assisted Reproductive Technology (ART). Accessed on: April 11, 2022. Available onlin4e at: https://icmr.org.in/index.php/national-registry-of-assisted-reproductive-technology-art
- Gupta P, Banker M, Patel P, Joshi B (2012) A study of recipient related predictors of success in oocyte donation program. J Hum Reprod Sci 5(3): 252-257.
- Purewal S, van den Akker OBA (2009) Systematic review of oocyte donation: investigating attitudes, motivations and experiences. Hum Reprod Update 15(5): 499-515.
- Anand G (2021) Assisted Reproductive Technology (Regulation) Bill, 2021: An Explainer - The Leaflet pp: 3.
- Gupta JA (2015) Reproductive biocrossings: Indian egg donors and surrogates in the globalized fertility market. 5(1): 25-51.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Veterinary and Marine Sciences (ISSN: 2689-7830)
- Journal of Agriculture and Forest Meteorology Research (ISSN:2642-0449)
- Journal of Genetics and Cell Biology (ISSN:2639-3360)
- Journal of Microbiology and Microbial Infections (ISSN: 2689-7660)
- Journal of Genomic Medicine and Pharmacogenomics (ISSN:2474-4670)
- Food and Nutrition-Current Research (ISSN:2638-1095)
- Journal of Biochemistry and Molecular Medicine (ISSN:2641-6948)


