2490
Views & Citations1490
Likes & Shares
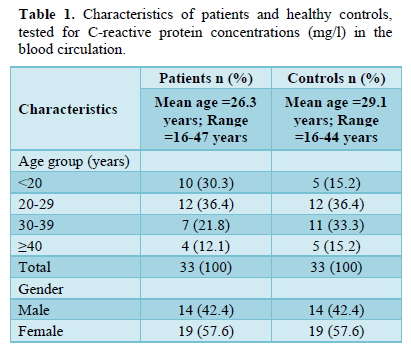
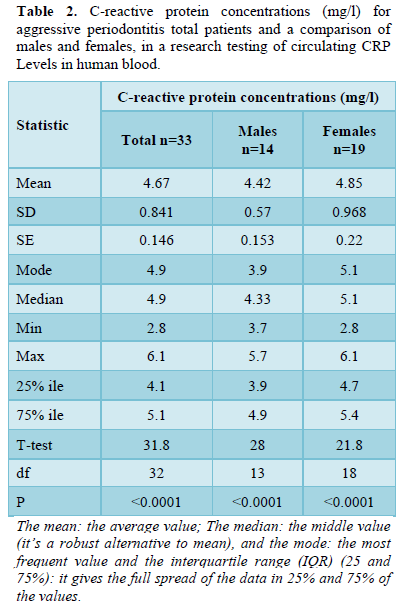
C-reactive protein concentrations (mg/l) for aggressive periodontitis total patients was as follow: the mean ± SD of circular C-reactive protein was 4.67 ± 0.841 mg/l, with a mode equal to 4.9 mg/L, the median was 4.9 mg/L, and ranged from 2.8 to 6.1 mg/l with the 75% interquartile range (IQR) equal to 5.1 mg/l; the variance in all individual values was significantly distributed on the normal curve with t-test of 31.8 and p < 0.0001.
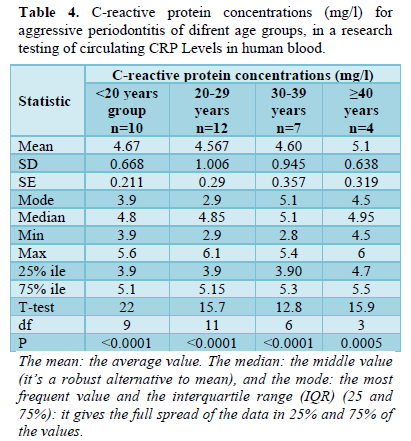
While for healthy controls the C-reactive protein concentrations (mg/l) were lower than that of the patients in which the mean ± SD of circular C-reactive protein was 2.067 ± 0.387 mg/l, with a mode equal to 2.1 mg/L, the median was 2.1 mg/L, and ranged from 1.2 to 3.1 mg/l with the 75% interquartile range (IQR) equal to 2.2 mg/l; the variance in all individual values was significantly distributed on the normal curve with t-test of 30.6 and p < 0.0001 (Table 3). Considering age groups effects on the C-reactive protein concentrations (mg/l) for aggressive periodontitis patients, roughly equal levels values were occurred in all age groups (Table 4).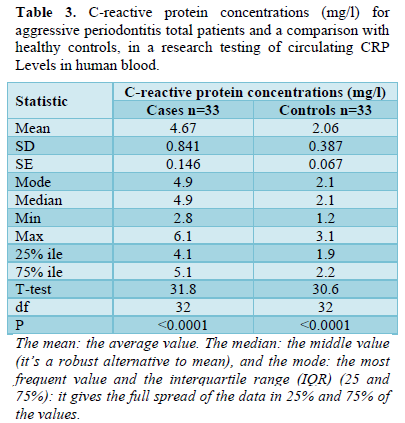
DISCUSSION
CRP represents a dependable marker of acute phase response to infectious burden and/or inflammation [35]. As a result of its kinetic possessions, C-reactive protein best illustrates the inflammatory state of the human [36]. Recent evidence indicated that patients with acute periodontitis had increased serum C-reactive protein levels compared to an unaffected control group [37]. We compared and evaluated systemic levels of CRP in peripheral blood samples of patients with aggressive periodontitis and healthy controls. CRP levels may fluctuate with various factors such as hypertension, alcohol use, smoking, diabetes, sleep disorders, chronic fatigue, depression, several other systemic diseases, and pregnancy or lactation [38]. Therefore, we established robust exclusion criteria (see Methods) for patients to be included in this study.
In the current study, the C-reactive protein (mg/L) concentrations of periodontitis patients were approximately 2.5 volumes higher than that of healthy controls (patients' mean ± SD = 4.67 ± 0.841 mg/L vs. 2.067 ± 0.387 mg/L of control). This underscores the fact that CRP levels correlate with severity of periodontal affection and aggressive periodontitis shows a stronger systemic inflammatory burden than the control group similar to that reported by Wohlfeil [39]. Also, our finding is similar to that study of Goyal [40] showed the highest CRP levels in patients with aggressive periodontitis and the lowest values in the group of healthy patients [40]. Other studies showed increased CRP levels in patients with chronic periodontitis compared to patients with gingivitis [41,42]. In a study by Podzimek [43] aggressive periodontitis patients had an average CRP of 2.8 mg/L below or levels of the same patient group (4.67 ± 0.841 mg/L). This difference may be explained by that, race has been found to influence CRP levels [44] and data in diverse populations are not comparable. A study from the USA reported C-reactive protein (CRP) levels of 2.05 mg/L in aggressive periodontitis patients with the generalized form and C-reactive protein (CRP) levels of 1.1 mg/L in patients with the localized form [45]. However, our result (4.67 ± 0.841 mg/L) is similar to another study from the USA that showed C-reactive protein (CRP) levels of 4.06 mg/L in subjects with high levels of clinical attachment loss mean [46].
While lower values were reported in a Swedish study showing an average CRP of 2 mg/L in periodontitis patients [47]. In the Netherlands, a study reported lower CRP levels than (1.45 mg/L) in patients with a generalized form of periodontitis and CRP levels of 1.30 mg/L in patients with the localized form [48]. Another study from India showed a higher level than our study where CRP levels of 7.49mg/L in aggressive periodontitis patients and CRP levels of 4.88mg/L in chronic periodontitis patients were found [49]. Therefore, in our study, only patients of Yemeni origin were included.
Regarding the statistical associations between male and female indices as well as ages with CRP levels determined in periodontitis patient groups, there were no significant differences with C-reactive protein concentrations (mg/L) for the comparison of aggressive periodontitis for males and females or for age groups (Tables 2 & 3). These results are similar to those previously reported in which no significant differences were found between sexes or between ages [43]. Thus, CRP increases with periodontal impairments. The CRP indicator is a very important issue. The observed association between periodontal conditions and systemic CRP demonstrated that periodontal affections may be a contributing factor to systemic inflammation. In the study by Beck [50]. CRP as a clinical parameter was more difficult to estimate the degree of systemic inflammation than traditional classifications of mild, moderate, and severe periodontitis or other measures of disease severity such as attachment loss [50]. The novelty of the results obtained was based on comparing one type of periodontal disease with healthy controls, as two or three types are usually compared, and on comparing various periodontal indices with subsequently determined CRP levels in the patient's peripheral blood.
CONCLUSION
Our study results show that CRP levels increase subsequently with the aggressive periodontitis patients, and there was no significant effect of sex or ages in the level. Further studies are needed to clarify this association and the associated confounding factors. In further research, other systemic markers that might be more specific to periodontal disease such as fibrinogen, leptin, white blood cell count, and interleukin-6 should be considered. Changes in their values during the treatment of periodontal disease could lead to improved monitoring of periodontal tissue status during therapy. Elevated levels of such markers of systemic inflammation are connected to both systemic diseases and periodontal diseases. Studying these markers would certainly be beneficial for monitoring periodontal disease therapy.
ACKNOWLEDGMENTS
The authors thank the Faculty of Dentistry, Sana'a University, Sana'a, and Yemen for their generous support.
CONFLICT OF INTEREST
No conflict of interest associated with this work.
- Newman MG, Takei HH, Klollevold PR, Carranza FA (2006) Classification of diseases and conditions affecting the periodontium. Clinical Periodontology. 10th Philadelphia: W.B. Saunders. pp: 103-104.
- Williams RC, Offenbacher S (2000) periodontal medicine. Periodontology 23: 9-16.
- Slade GD, Offenbacher S, Beck JD, Heiss G, Pankow JS (2000) Acute-phase inflammatory response to periodontal disease in the US population. J Dent Res 79: 49-57.
- Roberts FA, Darveau RP (2015) Microbial Protection and Virulence in Periodontal Tissue as a Function of Polymicrobial Communities: Symbiosis and Dysbiosis. Periodontology 69: 18-27.
- Hajishengallis G, Chavakis T (2021) Local and Systemic Mechanisms Linking Periodontal Disease and Inflammatory Comorbidities. Nat Rev Immunol 21(7): 426-440.
- Ebersole JL, Dawson D, Emecen-Huja P, Nagarajan R, Howard K, et al. (2017) The Periodontal War: Microbes and Immunity. Periodontology 75: 52-115.
- Botelho J, Machado V, Hussain SB, Zehra SA, Proença L, et al. (2020) Periodontitis and Circulating Blood Cell Profiles: A Systematic Review and Meta-Analysis. Exp Hematol 93: 1-13.
- Paraskevas S, Huizinga JD, Loos BG (2008) A Systematic Review and Meta-Analyses on C - reactive protein in Relation to Periodontitis. J Clin Periodontol 35: 277-290.
- Bansal T, Pandey A, Deepa D, Asthana AK (2014) C-Reactive Protein (CRP) and Its Association With Periodontal Disease: A Brief Review. J Clin Diagn Res 8: 21-24.
- Castro AB, Meschi N, Temmerman A, Pinto N, Lambrechts P, et al. (2017) Regenerative Potential of Leucocyte- and Platelet-Rich Fibrin. Part a: Intra-Bony Defects, Furcation Defects and Periodontal Plastic Surgery. A Systematic Review and Meta-Analysis. J Clin Periodontol 44: 67-82.
- Ioannidou E, Malekzadeh T, Dongari-Bagtzoglou A (2006) Effect of Periodontal Treatment on Serum C - reactive protein Levels: A Systematic Review and Meta-Analysis. J Periodontol 77: 1635-1642.
- de Freitas COT, Gomes-Filho IS, Naves RC, da Rocha Nogueira Filho G, da Cruz SS, et al. (2012) Influence of Periodontal Therapy on C-Reactive Protein Level: A Systematic Review and Meta-analysis. J Appl Oral Sci 20: 1-8.
- D’Aiuto F, Ready D, Tonetti MS (2004) Periodontal Disease and C-Reactive Protein- Associated Cardiovascular Risk. J Periodontal Res 39: 236-241.
- Abbas AM, Al-Kibsi TAM, Al-Akwa AAY, AL-Haddad KA, Al-Shamahy HA, et al. (2020) Characterization and antibiotic sensitivity of bacteria in orofacial abscesses of odontogenic origin. Univers J Pharm Res 5(6): 36-42.
- Al Makdad ASM, Al-Haifi AY, Salah MK, Al-Shamahy HA, Al-Falahi TH (2020) Urinary tract infections in post-operative patients: Prevalence rate, bacterial profile, antibiotic sensitivity and specific risk factors. Univers J Pharm Res 5(3): 21-26.
- Al-Akwa AA, Zabara A, Al-Shamahy HA, Al-labani MA, Al-Ghaffari KM. et al. (2020) Prevalence of Staphylococcus aureus in dental infections and the occurrence of MRSA in isolates. Univers J Pharm Res 5(2): 1-6.
- Al-dossary OAE, Al-Kholani AI, AL-Haddad K, Al-Najhi MM, Al-Shamahy HA, et al. (2022) Interleukin-1β levels in the human gingival sulcus: Rates and factors affecting its levels in healthy subjects. Univers J Pharm Res 7(5): 25-30.
- Al-Haddad KA, Al-dossary OE, Al-Shamahy HA (2018) Prevalence and associated factors of oral non-candida albicans candida carriage in denture wearers in Sana’a city- Yemen. Univers J Pharm Res 3(4): 7-11.
- AL-Haddad KA, Al-Najhi MM, Al-Akwa AAY, Al-Shamahy HA, Al-Sharani AA (2021) Antimicrobial susceptibility of Aggregatibacter actinomyce-temcomitans isolated from Localized Aggressive Periodontitis (LAP) Cases. J Dent Oral Health Adv Res 2: 1-6.
- Al-Haddad KA, Al-Najhi MMA, Abbas AKM, Al-Akwa AAY, Al-Shamahy HA, et al. (2021) Clinical features, age and sex distributions, risk factors and the type of bacteria isolated in periodontitis patients in Sana'a, Yemen. Univers J Pharm Res 6(1): 1-8.
- Al-Hajri MM, Al-Kadasi BA, Al-Wesabi MA, Aldeen HMS (2022) Gingival recession in relation to mucogingival deformities and other predisposing factors affect females in lower esthetic zone. Univers J Pharm Res 7(5): 50-55.
- Alhasani AH, Ishag RA, Al-Akwa AAY, Al Shamahy HA, Al-labani MA (2020) Association between the Streptococcus mutans biofilm formation and dental caries experience and antibiotics resistance in adult females. Univers J Pharm Res 5(6): 1-3.
- Al-Kebsi A, Othman A, Abbas AK, Madar E, Al-Shamahy HA (2017) Albicans colonization and non-Candida albicans Candida colonization among university students, Yemen. Univers J Pharm Res 2(5): 1-6.
- Al-Safani AA, Al-Shamahy H, Al-Moyed K (2018) Prevalence, antimicrobial susceptibility pattern and risk factors of MRSA isolated from clinical specimens among military patients at 48 medical compounds in Sana’a city-Yemen. Univers J Pharm Res 3(3): 40-44.
- Al-Sanabani NA, Al-Kebsi A, Al-Shamahy HA, Abbas A (2018) Etiology and risk factors of stomatitis among Yemeni denture wearers. Univers J Pharm Res 3(1): 1-6.
- Al-Shamahy HA, Abbas AMA, Mohammed AAM, Alsameai AM (2018) Bacterial and Fungal Oral Infections among Patients Attending Dental Clinics in Sana’a City-Yemen. Online J Dent Oral Health 1(1): 1-6.
- Al-Shamahy HA, Al-labani MA, Al-Akwa A (2020) Biofilm formation and antifungal susceptibility of candida isolates from oral cavity of denture wearer and free denture individuals. EC Dent Sci 19(10): 58-66.
- Al-Shami HZ, Al-Haimi MA, Al-dossary OAE, Nasher AAM, Al-Najhi MMA, et al. (2021) Patterns of antimicrobial resistance among major bacterial pathogens isolated from clinical samples in two tertiary’s hospitals, in Sana'a, Yemen. Univers J Pharm Res 6(5): 60-67.
- Al-Shami HIZ, Al-Shamahy HA, Majeed ALAA, Al-Ghaffari KM, Obeyah AA (2018) Association between the salivary Streptococcus mutans levels and dental caries experience in adult females. Online J Dent Oral Health 1(1): 1-6.
- Gylan EMA, Muharram BA, Al-Kholani AIM, AL-Haddad KA, Al-Akwa AAY, et al. (2022) In vitro evaluation of the antimicrobial activity of five herbal extracts against streptococcus mutans. Univers J Pharm Res 7(1): 1-6.
- Mutaher NJA, Al-Haddad KA, Al-Akwa AAY, Al-labani MA, Al-Shamahy HA, et al. (2020) Prevalence and causes of traumatic dental injuries to anterior teeth among primary school children in Sana’a city, Yemen. Univers J Pharm Res 5(3): 38-43.
- Al-Deen SH, Al-Ankoshy AAM, Al-Najhi MMA, Al-Shamahy HA (2021) Porphyromonas gingivalis: Biofilm formation, antimicrobial susceptibility of isolates from cases of Localized Aggressive Periodontitis (LAP). Univers J Pharm Res 6(4): 1-7.
- Newman M, Takei H, Klokkevold P, Carranza FA (2012) Carranza’s Clinical Periodontology, Elsevier Sauders, St. Louis, Mo, USA, 11th
- No Authors Listed (1999) 1999 International workshop for a classification of periodontal diseases and conditions. Papers. Oak Brook, Illinois, October 30-November 2, 1999. Ann Periodontol 4(1): 1-112.
- Ramamoorthy R, Nallasamy V, Reddy R, Esther N, Maruthappan Y (2012) A review of C-reactive protein: a diagnostic indicator in periodontal medicine. J Pharm Bioallied Sci 4(6): S422-S426.
- Gani D, Lakshmi D, Krishnan R, Emmadi P (2009) Evaluation of C-reactive protein and interleukin-6 in the peripheral blood of patients with chronic periodontitis. J Indian Soc Periodontol 13(2): 69-74.
- Gomes-Filho IS, Coelho JMF, da Cruz SS (2011) Chronic periodontitis and C-reactive protein levels. J Periodontol 82(7): 969-978.
- Graziani F, Cei S, Tonetti M (2010) Systemic inflammation following non-surgical and surgical periodontal therapy. J Clin Periodontol 37(9): 848-854.
- Wohlfeil M, Scharf S, Siegel Y (2012) Increased systemic elastase and C-reactive protein in aggressive periodontitis (CLOI-D-00160R2). Clin Oral Invest 16(4): 1199-1207.
- Goyal L, Bey A, Gupta ND, Sharma VK (2014) Comparative evaluation of serum C-reactive protein levels in chronic and aggressive periodontitis patients and association with periodontal disease severity. Contemp Clin Dent 5(4): 484-488.
- Jayaprakash D, Aghanashini S, Chatterjee A, Bharwani A, Vijayendra RR, et al. (2014) Effect of periodontal therapy on C-reactive protein levels in gingival crevicular fluid of patients with gingivitis and chronic periodontitis: A clinical and biochemical study. J Indian Soc Periodontol 18(4): 456-460.
- Shojaee M, Golpasha MF, Maliji G, Bijani A, Mir SMA, et al. (2013) C-reactive protein levels in patients with periodontal disease and normal subjects. Int J Mol Cell Med 2(3): 151-155.
- Podzimek S, Mysak J, Janatova T, Duskova J (2015) C-Reactive Protein in Peripheral Blood of Patients with Chronic and Aggressive Periodontitis, Gingivitis, and Gingival Recessions. Mediators Inflamm 2015: 1-7.
- Sun XJ, Meng HX, Shi D (2009) Elevation of C-reactive protein and interleukin-6 in plasma of patients with aggressive periodontitis. J Periodontal Res 44(3): 311-316.
- Salzberg TN, Overstreet BT, Rogers JD (2006) C-reactive protein levels in patients with aggressive periodontitis. J Periodontol 77(6): 933-939.
- Noack B, Genco RJ, Trevisan M (2001) Periodontal infections contribute to elevated systemic C-reactive protein level. J Periodontol 72(9): 1221-1227.
- Fredriksson MI, Figueredo CMS, Gustafson A, Bergström KG, Asman BE (1999) Effect of periodontitis and smoking on blood leukocytes and acute-phase proteins. J Periodontol 70(11): 1355-1360.
- Loos BG, Craandijk J, Hoek FJ (2000) Elevation of systemic markers related to cardiovascular diseases in the peripheral blood of periodontitis patients. J Periodontol 71(10): 1528-1534.
- Chopra R, Patil SR, Kalburgi NB, Mathur S (2012) Association between alveolar bone loss and serum C-reactive protein levels in aggressive and chronic periodontitis patients. J Indian Soc Periodontol 16(1): 28-31.
- Beck JD, Offenbacher S (2002) Relationships among clinical measures of periodontal disease and their associations with systemic markers. Ann Periodontol 7(1): 79-89.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Allergy Research (ISSN:2642-326X)
- Advance Research on Endocrinology and Metabolism (ISSN: 2689-8209)
- Journal of Carcinogenesis and Mutagenesis Research (ISSN: 2643-0541)
- Advance Research on Alzheimers and Parkinsons Disease
- Chemotherapy Research Journal (ISSN:2642-0236)
- BioMed Research Journal (ISSN:2578-8892)
- International Journal of Radiography Imaging & Radiation Therapy (ISSN:2642-0392)


