Case Report
Tinnitus in Mast Cell Activation Syndrome: A Prospective Survey of 114 Patients
48618
Views & Citations47618
Likes & Shares
Background: Tinnitus is a common idiopathic problem. Mast cell activation syndrome (MCAS) is a common multi-systemic disorder caused by unregulated mast cell (MC) activation resulting in allergic and inflammatory symptoms including the ears.
Objective: Determine prevalence and risk factors for tinnitus in MCAS.
Methods: Prospective analysis of tinnitus in newly diagnosed MCAS patients by means of a survey questionnaire and clinical history. MCAS diagnosis was based on MC symptoms in ≥2 organ systems plus ≥1: Elevated MC mediators, clinical improvement with MC-directed therapy, and/or increased intestinal MC density. Subjects were diagnosed after presenting with refractory gastrointestinal symptoms. Tinnitus prevalence was compared to a historical control group of the USA population with odds ratio (OR) calculation with 95% confidence interval (CI). Patient subgroups were compared using OR and Student’s t-tests with Cohen’s d for effect size.
Results: 114 MCAS subjects included 96 women and 18 men (mean age 45.8 years). Abnormal MC mediators were found in 73.7%. Prevalence of tinnitus was 61.4%, compared to 9.6% in the general United States population (OR 15.0; 95%CI 10.3 to 21.9; p<0.0001). Co-morbid conditions included postural orthostatic tachycardia syndrome (POTS)(25.4%), previously diagnosed fibromyalgia (20.2%), chronic fatigue with muscle pain (57.0%), migraines (33.3%) and headaches (67.3%).Increased risk of tinnitus was present in POTS (OR 3.1;95% CI 1.1 to 8.4; p=.027) and chronic muscle pain with fatigue(65.7% vs 43.2%;OR 2.5;95% CI 1.2 to 5.5;p=.021).
Conclusion: Tinnitus appears to be prevalent in MCAS. Co-morbid POTS and chronic muscle pain with fatigue are additional risks for tinnitus.
Keywords: Tinnitus, Mast cells, Mast cell activation syndrome, Prevalence, Allergy, Inflammation, Postural orthostatic tachycardia syndrome, Chronic fatigue, Muscle pain, Fibromyalgia
ABBREVIATIONS
BMI: Body Mass Index; CI: Confidence Intervals; F: Female; hpf: High Power Field; Inflam: Inflammation; M: Male; MC: Mast Cell; MCMRS: Mast Cell Mediator Release Syndrome; MCs: Mast Cells; N: Number; OR: Odds Ratio; POTS: Postural Orthostatic Tachycardia Syndrome; RR: Relative Risk; NS: Not Significant
INTRODUCTION
Tinnitus is a disruptive sound perception in the head without an external source [1]. Non-pulsatile tinnitus is most often idiopathic and results in poor quality of life [2]. In a review of epidemiological studies of tinnitus published in the decade 1993-2003, the authors found fourteen studies of prevalence data ranging from 3% to 30% [3]. In a more recent study, the prevalence of this auditory disorder in the United States was estimated to be 9.6% of the adult population, with slightly higher rates among males (10.5%) than females (8.8%) [4]. Risk factors for tinnitus include middle ear disease, depression, and cardiovascular disease [3].
Treatment options for tinnitus are limited and often do not appear to have a clear pharmacological rationale [5]. Interestingly, three commonly used tinnitus treatments (tricyclic antidepressants, benzodiazepines, and misoprostol) are also known to treat mast cell (MC) diseases including systemic mastocytosis and mast cell activation syndrome (MCAS) [6-8]. Additional risk factors for tinnitus include asthma and fibromyalgia which along with depression are common disorders seen in MCAS [9-12]. MCAS is a common yet often undiagnosed, unrecognized disorder with allergic and inflammatory symptoms [13]. MCAS has an estimated prevalence of 1% to 17%, yet is often not considered in the differential diagnosis of a multi-systemic disorder [14]. Autonomic dysfunction including postural orthostatic tachycardia (POTS) is common in MCAS and may be caused by autoimmune mechanisms or MC activation [15,16]. Theories are emerging that immune and autonomic dysfunction may also be present in patients with tinnitus [17,18].
MCAS features unregulated, aberrant MC activation leading to release of variable subsets of more than 1000 mediators identified to date as being produced by mast cells (MCs) [19]. Otolaryngologists often see undiagnosed MCAS patients owing to allergic manifestations (rhinitis, pharyngitis, and sinusitis) and inflammatory manifestations (oral sores, burning mouth syndrome and lymphadenopathy) [12,20,21]. In a retrospective study, 6.8% of 413 MCAS patients were affected by tinnitus; however, the histories of these patients were obtained in the course of routine clinical practice and a specific query regarding tinnitus was not performed in all of these patients (L.B. Afrin, personal communication 2020) [12]. A German study of MC activation disease patients demonstrated a higher rate of tinnitus compared to controls: 41/84 (48.8%) vs. 25/221 (11.3%); OR 7.7, 95% CI 4.3 to 13.8; p
METHODS
From May 2017 through April 2019, all consecutive adult patients presenting with refractory gastrointestinal (GI) symptoms to a gastroenterologist (LBW) who subspecializes in MCAS were evaluated for the presence of MCAS. Those who were subsequently diagnosed with MCAS were evaluated for the presence of tinnitus. The presence of tinnitus was based on a prospective questionnaire and confirmation by a clinical interview. Questions were asked to rule out specific tinnitus problems including prior damage by loud noise, pulsatile nature to the sounds, and vertigo-related tinnitus. Informed consent to collect data from subjects was obtained and the study was approved by the Missouri Baptist Medical Center Investigational Review Board in St. Louis, Missouri. The primary aim of the study was to examine all conditions that would lead to fatigue which is the most common symptom of MCAS. Sleep disturbance owing to restless legs syndrome and tinnitus were the main focuses of the research project. The data on restless legs syndrome was published in April 2020.
MCAS diagnosis was based on typical symptoms of MC activation in ≥2 organ systems plus ≥1 of: elevated MC mediator(s), clinical improvement with MC-directed therapy, and/or intestinal MC density ≥20 per high power field (HPF). MC mediator measurements included: 1) plasma prostaglandin D2 and histamine, 2) serum tryptase and chromogranin A, and 3) 24-hour urine 2,3 dinor 11-β-PGF2α, N-methylhistamine, and leukotriene E4 [23]. To confirm MC activation the MC mediator release syndrome (MCMRS) score was employed [22]. MCMRS is a questionnaire that assesses the number of symptoms, laboratory, radiographic and biopsy findings. A sum of 9 to 13 = a suspicion of a pathological activation of MCs as cause of the complaints. A sum ≥14 = clinical confirmation of a diagnosis of a MC mediator release syndrome. Mast cell density (MCD) was assessed by a standardized systematic approach where MCs were counted in multiple high-power fields (HPF) using Nikon BX41 Plan N 40x/0.65 objective magnification. MCD was expressed as a range including <20, 20-30, 31-40, 41-50, 51-60, 61-70, 71-80, 81-90, and 91-100 MC/HPF. The MCD was categorized as follows: 1) diffuse - MCD reported as least common denominator in any of several microscopic fields, 2) focal - highest MCMD reported in
Clinical data included age, gender, body mass index (BMI), medications, review of systems, past medical history, and the presence of postural orthostatic tachycardia syndrome (POTS) determined by past history and physical examination. The diagnosis of fibromyalgia was determined by review of records and prior history by a rheumatologist. In contrast, the diagnosis of chronic muscle pain with fatigue was determined by the questionnaire and clinical history. Chronic symptoms of hearing loss, nasal congestion, rhinitis, sore throat, allergic/infectious sinusitis and oral itching/irritation/burning/sores were queried. All patients underwent a standard physical examination and orthostatic pulse measurements.
Exclusion criteria in subjects included age under 18 years of age, pregnancy, history of loud noise exposure, pulsatile tinnitus and vertigo-related tinnitus.
The primary aim was to determine if prevalence of tinnitus was higher in MCAS subjects versus the general United States adult population [4]. Secondary aims included determination if there are MC mediators, MC density in biopsies, or medical disorders that increase the risk of tinnitus in MCAS patients. The prevalence of tinnitus in MCAS patients was compared to the general population with odds ratio (OR) calculation with 95% confidence interval (CI). Patient subgroups were compared using OR calculation and Student’s t-tests with Cohen’s d to describe effect size.
RESULTS
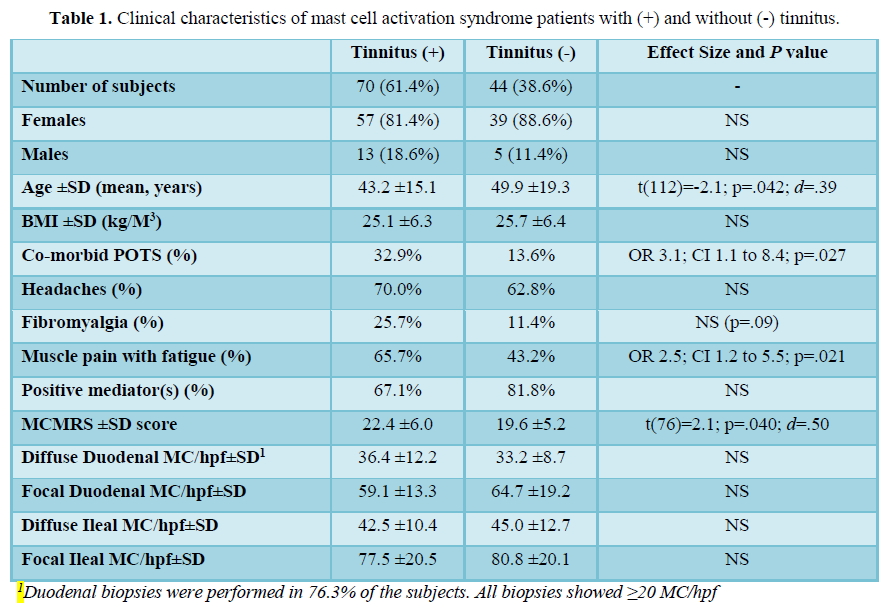
There were 114 MCAS subjects: 96F, 18 M, mean age 45.8 ±17.1 years, mean BMI 25.4±6.3 kg/m3. The subjects had co-morbid POTS in 25.4%. The clinical characteristics and analysis of risk factors for tinnitus are shown in Table 1. Chronic symptoms included: Nasal congestion (71.6%), rhinitis (59.5%), sore throat (48.3%), allergic/infectious sinusitis (47.4%), oral itching/irritation/burning/sores (43.1%) and hearing loss (36.2%).

Gender comparisons showed that female patients had significantly higher rates of POTS (p=0.03), migraines (p=0.0006), headaches (p=0.01), and chronic muscle pain with fatigue (p=.04). No other significant gender differences emerged.
Prevalence of tinnitus in 114 MCAS patients was 61.4%, compared to 9.6% in the general population (OR 15.0; 95% CI 10.3 to 21.9; p<0.0001). Co-morbid POTS was associated with higher risk of tinnitus. The rate of tinnitus in subjects with POTS was 79.3% versus 55.3% of those without POTS (OR 3.1; 95% CI 1.1 to 8.4; p=.027). Patients with tinnitus also had higher mean degree of systemic activity as determined by their MCMRS scores (22.4±6.0) vs. patients without tinnitus [19.6±5.2, t(76)=2.1, p=0.04, d=0.50]. Tinnitus-positive patients also had higher rates of chronic muscle pain with fatigue (65.7% vs. 43.2%; OR 2.5; 95% CI 1.2 to 5.5; p=0.021). Co-morbid migraines, headaches and previously diagnosed fibromyalgia were not different between groups.
Of the 114 subjects tested for MC mediators with urine and blood tests, 84 (73.7%) had one or more positive laboratory tests. The presence, number or type of MC mediators did not increase the risk for tinnitus. In 87 subjects who had intestinal biopsies, the median MC density was not associated with the risk for tinnitus.
DISCUSSION
The prevalence of tinnitus in MCAS in this study was 61.4% which is increased compared to the 9.6% prevalence in the general United States population [4]. Factors that increased the risk for tinnitus in MCAS included co-morbid POTS and chronic muscle pain with fatigue.
Theories to support the association of tinnitus and MCAS include the effect of inflammation, hypoxia and co-morbid autonomic dysfunction. Central neuroaudiological symptoms are seen in both fibromyalgia and MCAS [9,11,24]. There is evidence that demonstrates that fibromyalgia is associated with inflammatory mediators, including prostaglandins and IL-6, which are also produced by MCs [25,26]. Chronic muscle pain with fatigue with or without a formal diagnosis of fibromyalgia are among the most common problems in MCAS [12]. The impact of fibromyalgia and chronic muscle pain with fatigue on tinnitus prevalence in the present study was mixed, with fibromyalgia showing a trend toward association (p=0.09) and chronic muscle pain with fatigue being associated with greater risk of tinnitus (p=0.021). Abnormal MC activity alters the blood brain barrier, which is important to exclude inflammatory cell entry into the brain [26]. Theoretically, aberrant MC activity causes inflammation in the brain and also allows for passage of other inflammatory cells and cytokines into the brain leading to neurologic disorders including tinnitus [24,25].
Hypoxia could play a role in both tinnitus and MCAS. Koca et al. examined the clinical nature of tinnitus, hearing, vertigo and balance complaints and levels of serum glutathione peroxidase, nitric oxide, and malondialdehyde concentrations in fibromyalgia patients Neuroaudiological symptoms and oxidative stress markers were significantly increased in fibromyalgia vs. controls [11]. Oxidative stress and hypoxia is also seen with activated MCs [27].
Autonomic dysfunction, especially POTS, is a common co-morbid condition of MCAS [16]. In the present study, POTS was associated with higher risk of having tinnitus. Tinnitus has been linked to autonomic dysfunction, and preliminary evidence suggests that treatment of autonomic dysfunction can reduce the severity of tinnitus [17,18,28]. A recent case report of a patient with severe, daily tinnitus for 15 years had immediate and permanent relief of the tinnitus using intravenous immune globulin to treat her dysautonomia and MCAS [29].
Limitations of this study include lack of a local age, gender matched control group, the Tinnitus Functional Index was not employed, an audiogram was not performed, and the physical exam did not include listening around the ear and neck for sounds similar to the tinnitus and palpation of the craniocervical musculature for trigger points. Another criticism might include the fact that these patients were diagnosed with MCAS by a gastroenterologist for refractory GI symptoms, yet this scenario is extremely common for the undiagnosed MCAS patient who often presents to numerous physicians addressing system-wide complaints [30]. Finally, although specific MC mediators did not correlate with a risk for tinnitus, this is not unexpected. The MC is capable of secreting over 1000 mediators. Commercial labs are able to measures only a few which are relatively specific to MCs, including, histamine, prostaglandins, leukotrienes, heparin, chromogranin, and tryptase. It is worth noting that tryptase is elevated in only 15% of MCAS patients, with one round of testing only 50-75% of patients will demonstrate increased mediators, and the highest yield test (heparin) requires a highly sensitive assay [12,23,30].
CONCLUSION
Tinnitus appears to be prevalent in MCAS, which is a common, often undiagnosed syndrome. Inflammation, hypoxia and/or dysautonomia associated with MCAS may play pathophysiologic roles. It is paramount to look for underlying treatable causes for tinnitus. Symptomatic response of tinnitus to inhibitors of MC activation or MC mediator production could lead to new avenues in therapy of apparent idiopathic tinnitus.
- Piccirillo JF, Rodebaugh TL, Lenze EJ (2020) Tinnitus. JAMA 323(15): 1497-1498.
- Levine RA, Oron Y (2015) Tinnitus. Clin Neurol 129: 409-431.
- Sanchez L (2004) The epidemiology of tinnitus. Audiol Med 2: 18-17.
- Bhatt JM, Lin HW, Bhattacharyya N (2016) Prevalence, severity, exposures and treatment patterns of tinnitus in the United States. JAMA Otolaryngol Head Neck Surg 142(10): 959-965.
- Zenner HP, Delb W, Herwig BK, Jäger B, Peroz I, et al. (2017) A multidisciplinary systematic review of the treatment for chronic idiopathic tinnitus. Eur Arch Oto-Rhino-L 274: 2079-2091.
- Briner W, House J, O'Leary M (1993) Synthetic prostaglandin e1 misoprostol as a treatment for tinnitus. Arch Otolaryngol Head Neck Surg 119: 652-654.
- Baldo P, Doree C, Molin P, Don MF, Cecco S (2012) Antidepressants for patients with tinnitus. Cochrane Database Syst Rev 2012(9): CD003853.
- Jufas NE, Wood R (2015) The use of benzodiazepines for tinnitus: Systematic review. J Laryngol Otol 129 (3): S14-22.
- Bayazıt YA, Gürsoy S, Özerb E, Karakurum G (2002) Neurotologic manifestations of the fibromyalgia syndrome. J Neurol Sci 196: 77-80.
- Kim HJ, Lee HJ, An SY, Sim S, Park B, et al. (2015) Analysis of the prevalence and associated risk factors of tinnitus in adults. PLoS One 10(5): e0127578.
- Koca TT, Seyithanoglu M, Sagiroglu S, Berk E, Dagli H (2018) Frequency of audiological complaints in patients with fibromyalgia syndrome and its relationship with oxidative stress. Niger J Clin Pract 21: 1271-1277.
- Afrin LB, Self S, Menk J, Lazarchick J (2017) Characterization of mast cell activation syndrome. Am J Med Sci 353: 207-215.
- Afrin LB, Butterfield JH, Raithel M, Molderings GJ (2016) Often seen, rarely recognized: Mast cell activation disease--a guide to diagnosis and therapeutic options: Review article. Ann Med 48: 190-201.
- Afrin LB, Molderings GJ (2014) A concise, practical guide to diagnostic assessment for mast cell activation disease. World J Hematol 3: 1-17.
- Shibao C, Arzubiaga C, Robertsll LJ, Raj S, Black B, et al. (2004) Hyperadrenergic postural tachycardia syndrome in mast cell activation disorders. Hypertension 45: 385-390.
- DiBaise JK, Harris LA, Goodman B (2018) Postural Tachycardia Syndrome (POTS) and the GI Tract: A primer for the gastroenterologist. Am J Gastroenterol 113: 1458-1467.
- Choi EJ, Yun Y, Yoo S, Kim KS, Park JS, et al. (2013) Autonomic conditions in tinnitus and implications for Korean medicine. Evid Based Complement Alternat Med 2013: 402585.
- Kim SH, Kim JY, Lee HJ, Gi M, Kim BG, et al. (2014) Autoimmunity as a candidate for the etiopathogenesis of Meniere's disease: Detection of autoimmune reactions and diagnostic biomarker candidate. PLoS One 9(10): e111039.
- Ibelgaufts H (2020) Mast cells in cytokines & cells online pathfinder encyclopaedia. Version 50.9. Available online at: www.CellsTalk.com
- Pawankar R, Lee KH, Nonaka M, Takizawa R (2007) Role of mast cells and basophils in chronic rhinosinusitis. Clin Allerg Immunol 20: 93-101.
- Afrin LB (2011) Burning mouth syndrome and mast cell activation disorder. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 111: 465-472.
- Molderings GJ, Haenisch B, Bogdanow M, Fimmers R, Nöthen MM (2013) Familial occurrence of systemic mast cell activation disease. PLoS One 8(9): e76241.
- Afrin LB, Ackerley MB, Bluestein LS, Brewer JH, Brook JB, et al. (2020) Diagnosis of mast cell activation syndrome: A global “consensus-2.” Diagnosis. Epub ahead of print. Available online at: DOI: 10.1515/dx-2020-0005
- Afrin LB, Pöhlau D, Raithel M, Haenisch B, Dumoulin FL, et al. (2015) Mast cell activation disease: An underappreciated cause of neurologic and psychiatric symptoms and diseases. Brain Behav Immun 50: 314-321.
- Kadetoff D, Lampa J, Westman M, Andersson M, Kosek E (2012) Evidence of central inflammation in fibromyalgia - Increased cerebrospinal fluid interleukin-8 levels. J Neuroimmunol 242: 33-38.
- Ribatti D (2013) The crucial role of mast cells in blood-brain barrier alterations. Exp Cell Res 228: 119-125.
- Liang X, Yin G, Ma Y, Xu K, Liu J, et al. (2016) The critical role of mast cell-derived hypoxia-inducible factor-1α in regulating mast cell function. J Pharm Pharmacol 68: 1409-1416.
- Vanneste S, De Ridder D (2012) Noninvasive and invasive neuromodulation for the treatment of tinnitus: An overview. Neuromodulation 15: 350-360.
- Weinstock LB, Brook JB, Myers TL, Goodman B (2018) Successful treatment of postural orthostatic tachycardia and mast cell activation syndromes using naltrexone, immunoglobulin and antibiotic treatment. BMJ Case Rep 2018: bcr-2017-221405.
- Weinstock LB, Pace LA, Rezaie A, Afrin LB, Molderings GJ (2020) Mast Cell Activation Syndrome: A primer for the gastroenterologist. Dig Dis Sci. Epub ahead of print. Available online at: DOI: 10.1007/s10620-020-06264-9.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Neurosurgery Imaging and Techniques (ISSN:2473-1943)
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)
- Journal of Rheumatology Research (ISSN:2641-6999)
- Journal of Carcinogenesis and Mutagenesis Research (ISSN: 2643-0541)
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)
- Journal of Allergy Research (ISSN:2642-326X)
- Archive of Obstetrics Gynecology and Reproductive Medicine (ISSN:2640-2297)


