Research Article
Mortality and Comorbidities of Tuberculosis in Rheumatoid Arthritis A Retrospective Clinicopathologic Study of 161 Autopsy patients
4237
Views & Citations3237
Likes & Shares
Tuberculosis (TB) is one of the most important diseases accompanying rheumatoid arthritis (RA).The aim of this study was to determine the prevalence and mortality of post-primary fibrous or fibrocaseous TB (fTB or fcTB) with or without miliary dissemination (mTB), to analyze the relationship between fTB, fcTB or mTB and mortality or clinical diagnosis of TB, and to assess the possible influence of fTB, fcTB or mTB on the prevalence and mortality of comorbidities: atherosclerosis (Ath), hypertension (HT) or adult type 2 diabetes mellitus (DM) in a random autopsy population of RA patients.
Patients (autopsy population) and methods: At the National Institute of Rheumatology 9475 patients died between 1969 and 1992; among them 161 with RA which were autopsied. RA was confirmed clinically according to the criteria of the American College of Rheumatology (ACR). TB was detected at autopsy and specified histologically, all available clinical and pathological reports were retrospectively reviewed. Demographics of different patient cohorts were compared with the Student (Welch) t-probe.
The relationships were analyzed between fTB, fcTB, mTB and comorbidities (Ath, HT or DM) with Pearson's chi-squared (χ2) test.
Results: Post-primary TB localized to the lungs accompanied RA in 21 (13.04% of 161) patients. TB was inactive fTB or fcTB in 15 RA patients and was complicated with active military dissemination (mTB) in further 6 patients. fcTB complicated by mTb led to death in 2 female patients; the cases were not recognized clinically (clinically only 2 not fatal fTB was diagnosed). The chance of survival decreased significantly in RA patients with fcTB or mTB; the fTB did not influence statistically the lifespan of RA patients. The link between clinical diagnosis and mortality of TB was not significant, indicating the incidental nature of diagnosis. TB (fTB, fcTB or mTB) and comorbidities (Ath, HT or DM) were sovereign associated diseases in RA, which were present at the same time; TB and Ath led to death independently from each other.
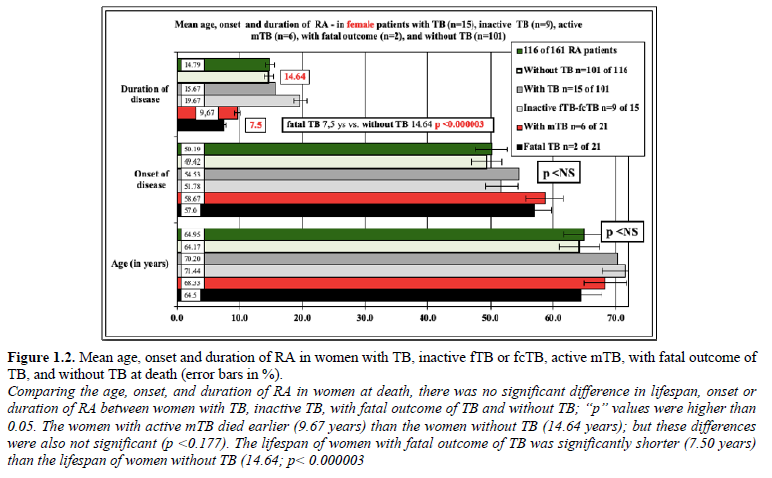
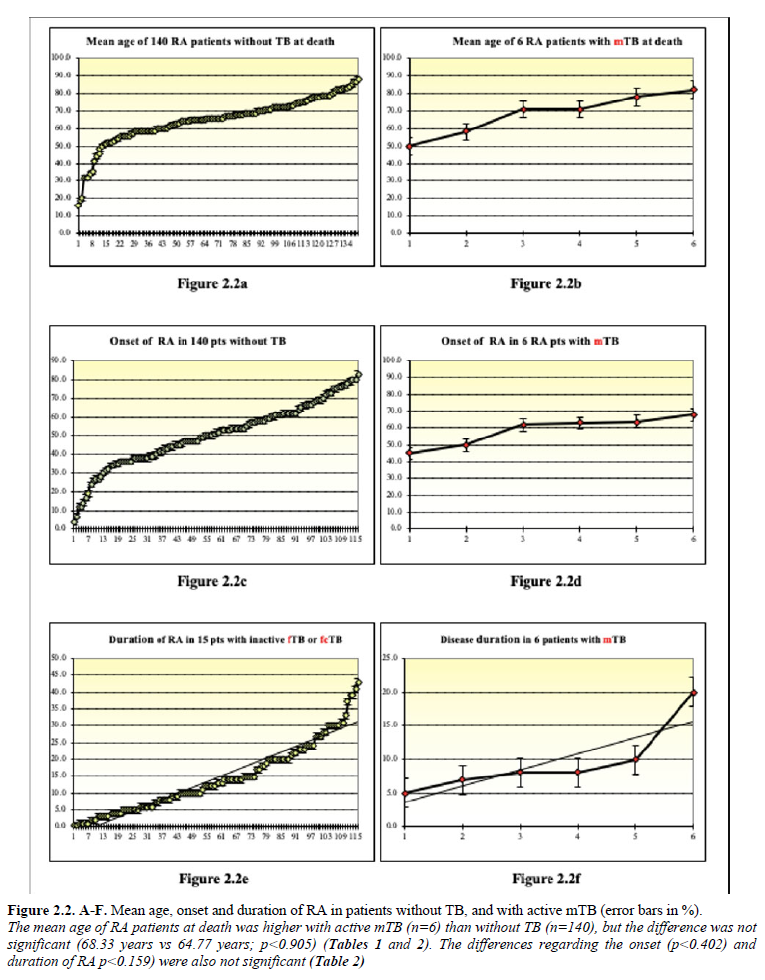
Discussion and Conclusions: The prevalence of post-primary TB was higher in RA autopsy patients compared to the general population of Hungary. Inactive fTB or fcTB (n=15) and active mTB (n=6) developed at any time in the course of RA. The presence of fcTB or mTB did increase the risk of mortality of RA patients, while consolidated anthracotic scars (fTB) did not. Women were more likely to be affected by TB in RA, in contrast to to the European general population, where more males died of TB, than females. The risk of active miliary dissemination (mTB) and fatal outcome was particularly high in women, who died earlier then women without mTB. The onset, and duration of RA did not influence the prevalence and mortality of inactive or active TB.
Keywords: Rheumatoid arthritis, Latent tuberculosis, Mortality, Clinical diagnosis, Comorbidities
ABBREVIATIONS
RA: Rheumatoid Arthritis; ACR: American College of Rheumatology; TB: Tuberculosis; fTB: Fibrous TB; fcTB: Fibrocaseous TB; mTB: Miliary TB; csDMARDs: Conventional Synthetic Disease Modifying Antirheumatic Drugs; bDMARDs or boDMARDs: Biological Original Disease Modifying Antirheumatic Drugs; Ath: Atherosclerosis; HT: Hypertension; DM: Diabetes Mellitus; RhV: Rheumatoid Vasculitis; AAa: Amyloid A Amyloidosis; SI: Septic Infection; PA: Purulent Arthritis; Pr n/y – Protocol Number /Year; Cl+: Clinically Diagnosed; Cl-: Clinically Not Recognized; SD: Standard Deviation; ND: No Data; NS: Not Significant; HE: Hematoxylin-Eosin Staining; c – Coefficient of Colligation; IGRAs: Interferon-Gamma (γ) Release Assays
INTRODUCTION
Tuberculosis (TB) is one of the most important diseases accompanying rheumatoid arthritis (RA) beside atherosclerosis (Ath), hypertenson (HT), and adult type 2 diabetes mellitus (DM) [1]. The risk of TB is higher in patients with RA than in the general population [2 – 6] and especially high when the patients are treated with steroids, and conventional or biological disease-modifying antirheumatic drugs (cDMARDs or bDMARDs) [7].
Introduction of bDMARDs (TNF inhibitors, etc.) increases the risk of reactivation of dormant TB in RA [8–9].
OBJECTIVE
The subject of this study was to assess the risk of clinically latent (not diagnosed) indolent TB in RA in the era of steroid and cDMARDs, before introduction of the bDMARDs. The aim was to determine the prevalence and mortality of post-primary inactive fibrous or fibrocaseous TB (fTB or fcTB) with or without miliary dissemination (mTB), to analyze the relationship between fTB, fcTB or mTB and mortality or clinical diagnosis of TB, to assess the possible influence of fTB, fcTB or mTB on the prevalence and mortality of comorbidities (Ath, HT or DM) in a non selected (random) autopsy population of RA patients.
Patients (autopsy population) and methods
Of special significance is the fact that until the end of the 20th century all patients who died in a hospital in Hungary were autopsied. This study is based on this unique autopsy population of our Pathology Department [1]. At the National Institute of Rheumatology 9475 patients died between 1969 and 1992; among them 161 non-selected patients with RA. All patients who died with RA were treated in one institute in an era of steroid and csDMARDs, before introduction of bDMARDs. RA was confirmed clinically according to the criteria of the American College of Rheumatology (ACR) [10]. TB was detected at autopsy and specified histologically, retrospectively reviewing all available clinical and pathological reports. From each patient a total of 50-100 tissue blocks of 16 organs (heart, lungs, liver, spleen, kidneys, pancreas, gastrointestinal tract, adrenal gland, skeletal muscle, peripheral nerve, skin, brain, bone and synovial membrane of hip- and knee-joint) were studied microscopically [1].
The tissue blocks were fixed in an 8% aqueous solution of formaldehyde at pH 7.6 for >24 hours at room temperature (20 Co) and embedded in paraffin. Serial sections (5 microns) were stained with hematoxylin-eosin (HE) [11]. In case of fTB, fcTB or mTB additional histological sections were stained according to Ziehl-Neelsen [12] using a positive control in each case. Atherosclerosis (Ath) was diagnosed in RA patients only when it was present macroscopically as a “severe” atherosclerotic process (characterized by occlusive thrombosis or sclerotic ulcers) or, when it was the basic disease leading to death. Moderate changes like hyaline or sclerotic plaques – without causal role in death – were not mentioned as “atherosclerosis”; such changes are frequent in elderly RA patients. The diagnosis of HT and DM were based on clinical data.
Demographics of different patient cohorts were compared with the Student (Welch) t-probe [13]. The difference between two samples was regarded “significant” at an alpha level of 0.05. The relationships were analyzed with person’s chi-squared (c²) test [13] comparing fTB, fcTB or mTB and mortality of TB, fTB, fcTB or mTB and clinical diagnosis of TB, furthermore fTB, fcTB or mTB and prevalence and mortality of comorbidities (Ath, HT or DM) in RA.
RESULTS
Prevalence of TB, fTB, fcTB or mTB
Post-primary fTB (n=12) or fcTB (n=9) localized to the lungs accompanied RA in 21 (13.04% of 161) patients.
Post-primary fTB (n=12) or fcTB (n=9) localized to the lungs accompanied RA in 21 (13.04% of 161) patients.
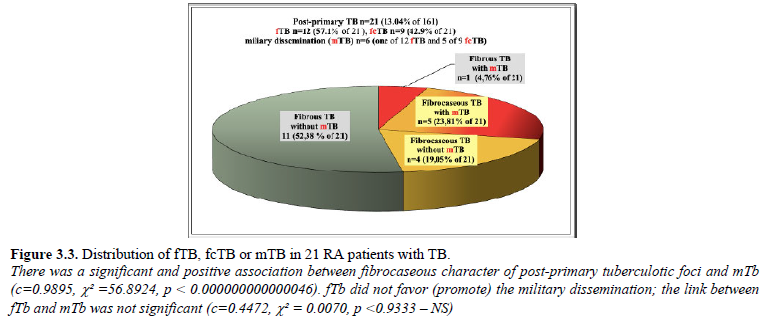
One of 12 fTB and 5 of 9 fcTB were complicated by disseminated mTB in 6 (3.7% of 161; 28.57% of 21) RA patients. Miliary epitheloid granulomatous inflammation was not observed without post-primary fTB or fcTB.
There was a significant and positive association between fibrocaseous character of post-primary tuberculotic foci and mTb (c=0.9895, c² =56.8924, p < 0.000000000000046). fTb did not favor (promote) the military dissemination; the link between fTb and mTb was not significant (c=0.4472, c² = 0.0070, p
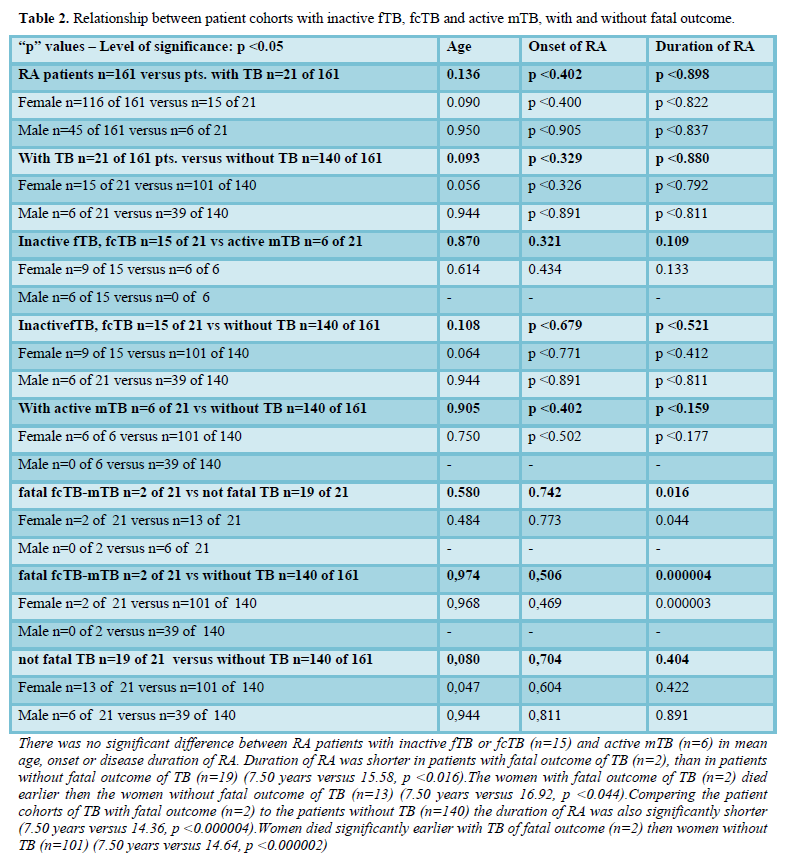
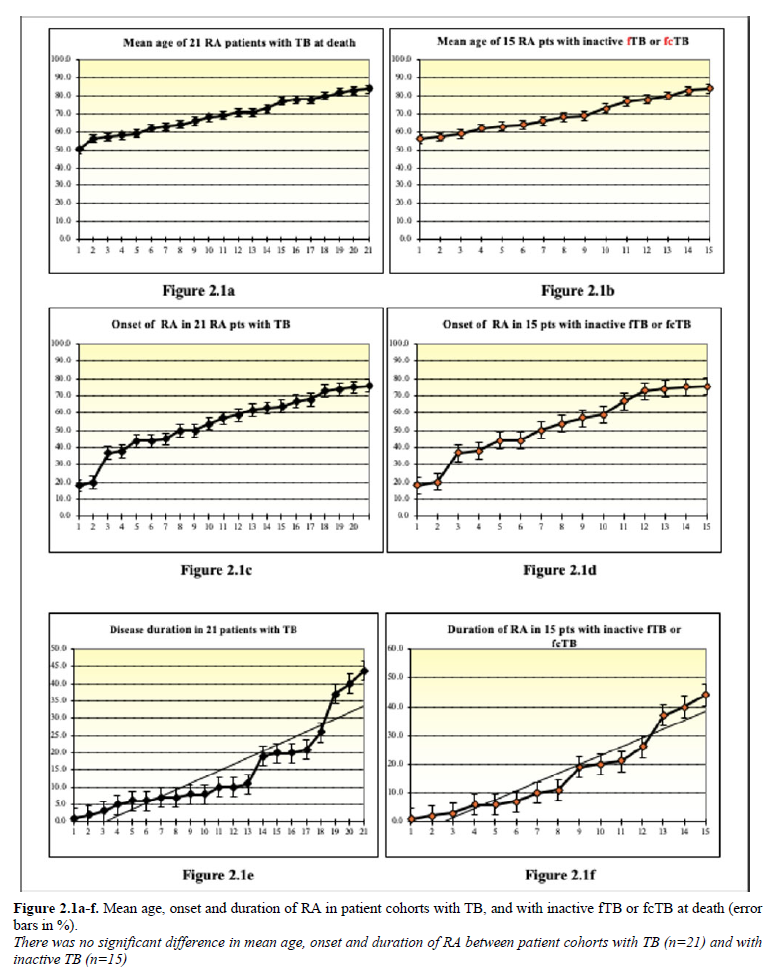
Table 1 summarizes the demographics, onset and duration of RA in patients with TB (n=21), inactive fTB or fcTB (n=15) and active mTB (n=6), with (n=2) and without (n=19) fatal outcome, furthermore without TB (n=140).
Comparing the age, onset, and duration of RA at the time of death there was no significant difference in lifespan (p

Figure 1.1 demonstrates the mean age, onset and duration of RA in patient cohorts, and Figure 1.2 in women with TB (n=21), inactive fTB or fcTB (n=15), active mTB (n=6), with fatal outcome of TB (n=2), and without TB (n=140) at death.
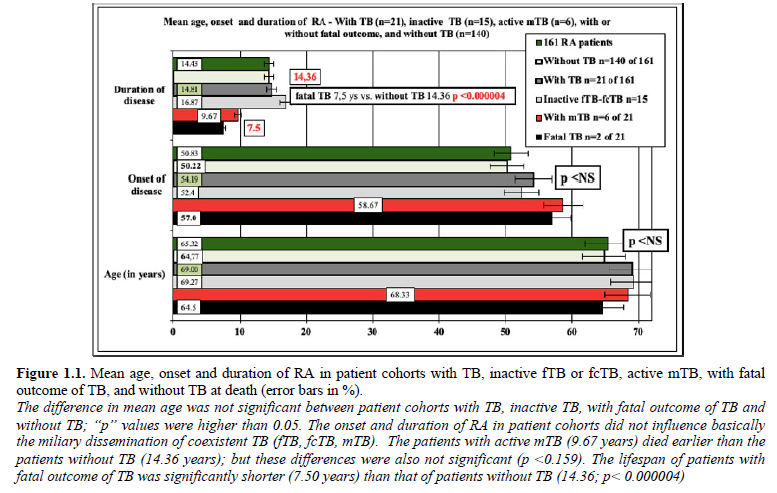
Table 2 summarizes the statistical correlations (“p” values) between female and male RA patients with inactive fTB, fcTB and active mTB, with and without fatal outcome.
Figures 2.1a-f – 2.2a-f demonstrates the mean age, onset and duration of RA in patient cohorts with TB (n=21), inactive fTB or fcTB (n=15), active mTB (n=6), and without TB (n=140) at death.
Mortality of TB, fTB, fcTB or mTB
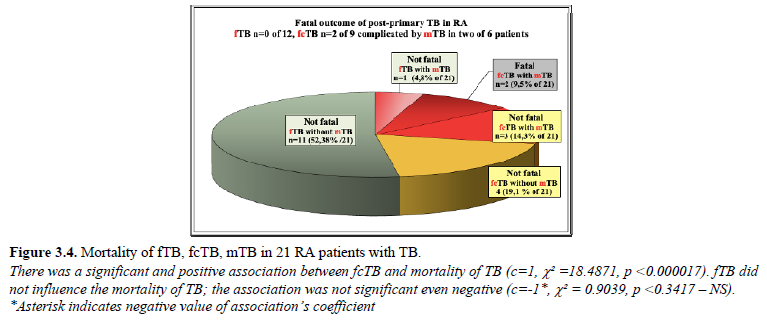
fcTB complicated by mTb led to death in 2 (9.52% of 21; 1.24% of 161) female patients; TB without mTb was not fatal. There was a significant correlation between TB and mortality of TB (c=1, c²=6.8539, p<0.009), fcTB and mortality of TB (c=1, c²=18.4871, p<0.000017) or mTB and mortality of TB (c=1, c²=28.6735, p<0.00000009); the chance of survival decreased significantly in RA patients with TB, fcTB or mTB. There was no relation between fTB and mortality of TB (c=-1*, c²=0.9039, p





Clinical diagnosis of TB, fTB, fcTB or mTB
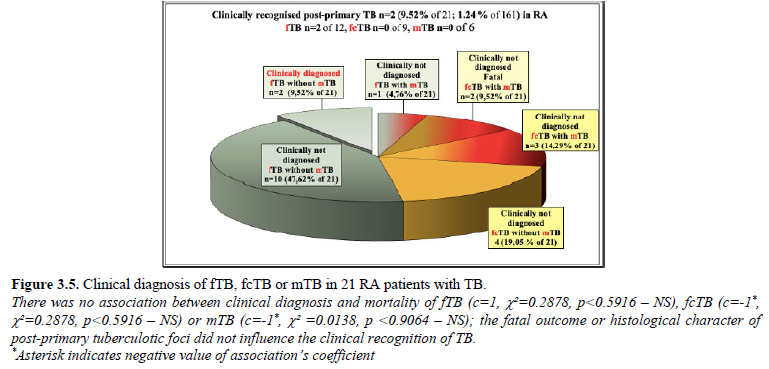
fTB was mentioned only in 2 (9.52% of 21; 1.24% of 161) patients with consolidated fibrous tubercular scars (the two cases of fcTB complicated by mTB with fatal outcome were not diagnosed clinically). fTB or fcTB was clinically latent (not recognized and/or not mentioned in clinical reports) in 19 (90.48% of 21; 11.80% of 161) patients. The links between clinical diagnosis and mortality of fTB (c=1, c²=0.2878, p*, c²=0.2878, p*, c²=0.0138, pTable 3 and Figures 3.1-3.5.
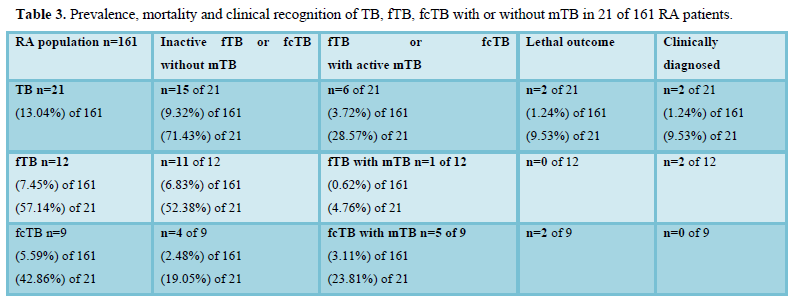
Figure 3.1 demonstrates the distribution of inactive fTB or fcTB (n=15) and active mTB (n=6) in 21 RA patients with TB.
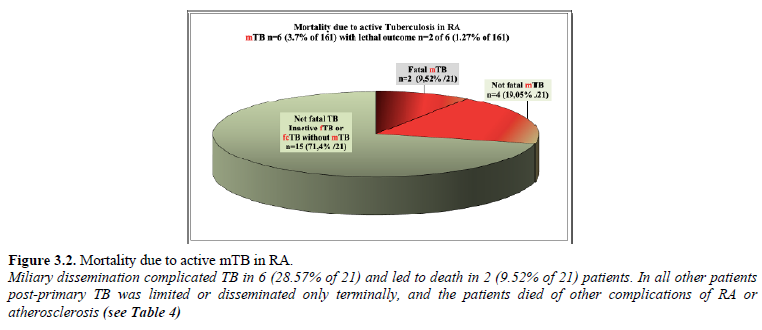
Mortality of TB with miliary dissemination (mTB) (n=2 of 6) is demonstrated in Figure 3.2.
Figure 3.3 demonstrates the prevalence of fTB or fcTB post-primary tuberculotic foci in the lungs with or without miliary dissemination (mTB) in 21 RA patients with TB.
Figure 3.4 demonstrates the distribution of fTB, fcTB or mTB with or without fatal outcome in 21 RA patients with TB.
Figure 3.5 demonstrates the clinical diagnosis of fTB, fcTB or mTB with or without fatal outcome in 21 RA patients with TB.






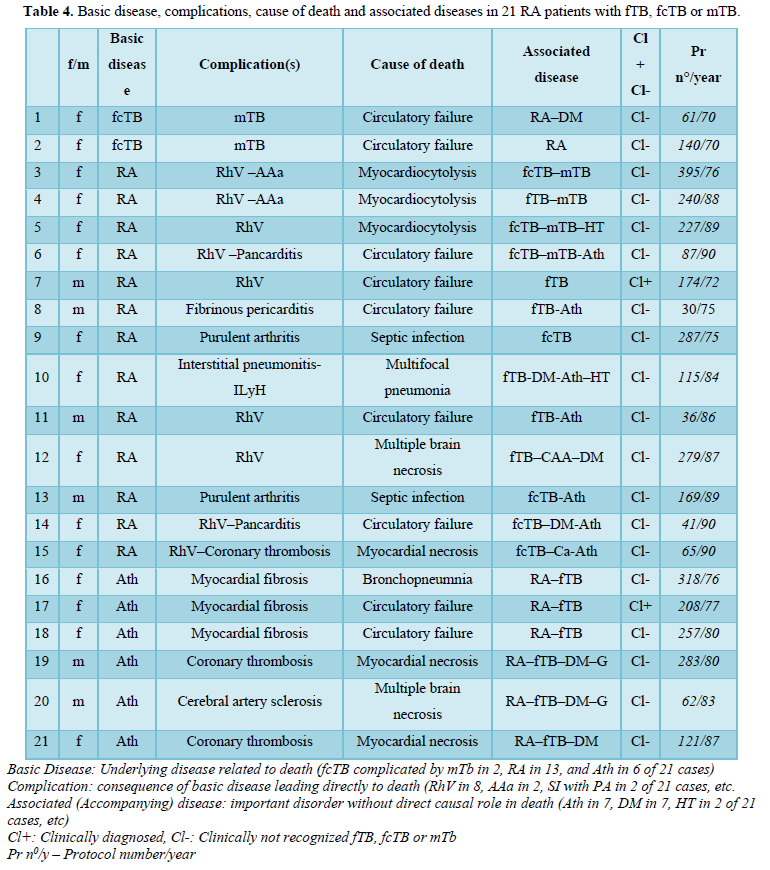
Basic diseases, complications and accompanied disorders in 21 RA patients with fTB, fcTB or mTB
In our autopsy population 2 (1.24% of 161) RA patients died of circulatory failure due to fibrocaseous tuberculosis complicated by miliary dissemination; in one case the extended miliary dissemination and in the second one the involvement of the pituitary gland and collapsed regulation system led to circulatory insufficiency and fatal outcome (Tables 1 and 2). The hematogenous dissemination of post-primary TB was limited in all other patients and involved only a few organs; the patients died of other complications of basic diseases: RA or atherosclerosis (Ath) (Table 4). The most important complications and associated diseases in 21 RA patients with fTB, fcTB or mTB, and the clinical recognition of TB are summarized in Table 4.
Ath* was diagnosed in RA patients only when it was present macroscopically as a “severe” atherosclerotic process or when it was the basic disease leading to death. HT** only atherosclerosis related hypertension was listed; RA related hypertension caused by glomerulonephritis or renal AAa, RhV, Cushing syndrome, etc. were not considered. The diagnosis of HT and DM*** was based on clinical data.
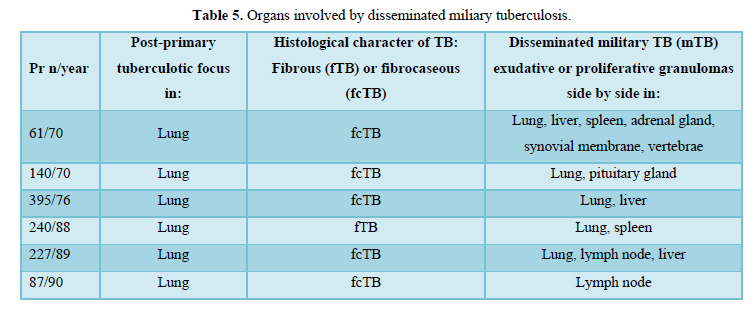
Proliferative or exudative epithelioid granulomas (mTB) existed side by side in the same or in different organs, such as lungs, liver, spleen, adrenal glands, synovial membrane, vertebrae, pituitary gland, and lymph nodes.
The organs involved by disseminated military tuberculosis are listed in Table 5.
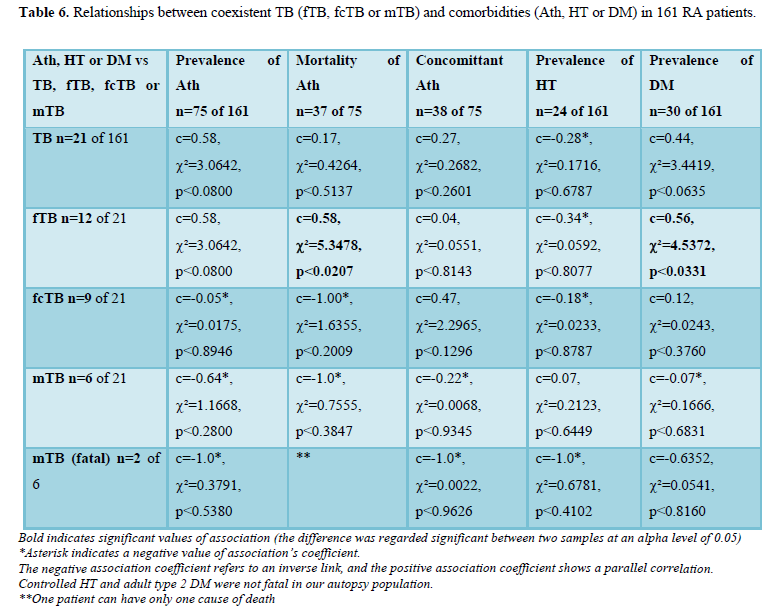
Possible influence of TB (fTB, fcTB or mTB) on comorbidities (Ath, HT or DM) of RA with and without lethal outcome
Atherosclerosis (Ath) accompanied RA in 75 (46.58%) of 161 patients and led directly to death in 37 (22.98% of 161 and 49.33% of 75) cases; Ath in 38 (23.60% of 161 and 50.6 7% of 74) cases was only concomitant (associated disease) without direct causal role in death. TB was associated with Ath in 13 of 75 patients: fTB in 9, fcTB in 4 (one of them complicated by mTB) cases. TB was associated with fatal Ath in 6 patients of 37 patients: fTB in 6, fcTB or mTB in none of 37 patients. TB was associated with concomitant Ath in 7 of 38 patients: fTB in 3, fcTB in 4 (one of them complicated by mTB) cases. The correlations were not significant between TB (fTB, fcTB or mTB) and prevalence of Ath (n=75), TB (fcTB or mTB) and mortality of Ath (n-37) or TB (fTB, fcTB or mTB) and concomitant Ath (n=38). There was a positive and significant correlation betveen fTB and mortality of Ath (c=0.58, c²=5.3478, p<0.0207). Hypertension (HT) was observed in 24 (14.91%) of 161 RA patients; the HT was controlled in all cases and did not lead to death. TB was assotiated with HT in 2 of 24 patients: fTB in 1, fcTB in 1 case (complicated by mTB). The correlations were not significant between TB (fTB, fcTB or mTB) and HT. Adult type 2 diabetes mellitus (DM) was found in 30 (18.63%) of 161 RA patients; the DM was controlled in all cases and did not lead to death. TB was assotiated with DM in 7 of 30 patients: fTB in 5, fcTB in 2 (in one case complicated by mTB). The correlations were not significant between TB (fcTB or mTB) and DM, except fTB and DM, where the relationship was positive and significant (c=0.56, c²=4.5372, p<0.0331). Fatal mTB (n=2) was associated with DM in one of two patients and was not with Ath or HT; the correlations were not significant. The statistical links between TB (fTB, fcTB or mTB) and comorbidities (Ath, Hy or DM) are summarized in Table 6.
Figures 4 and 10 demonstrate TB (fTB, fcTB, mTB) with traditional HE is staining, viewed by light microscopy. Original magnifications correspond to the 24x36 mm transparency slide; the correct height: width ratio is 2:3. The printed size may be different; therefore, it is necessary to indicate the original magnifications.
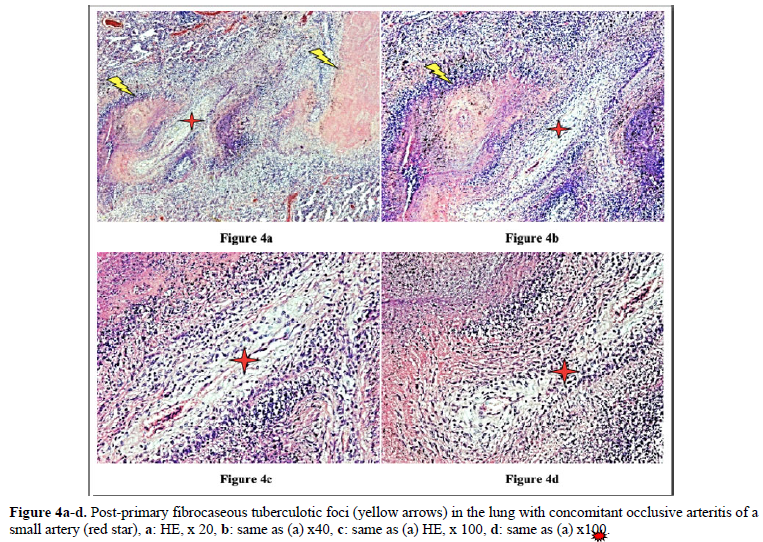
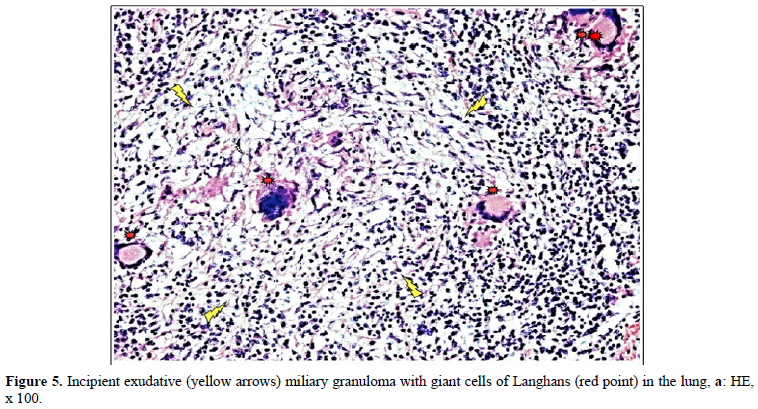
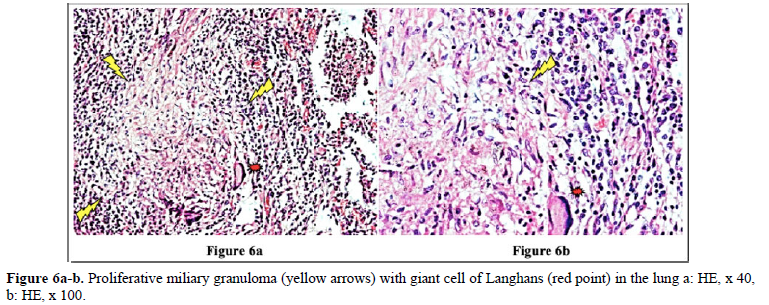
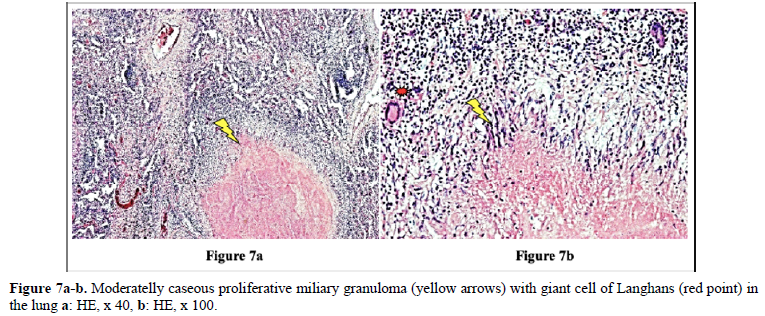
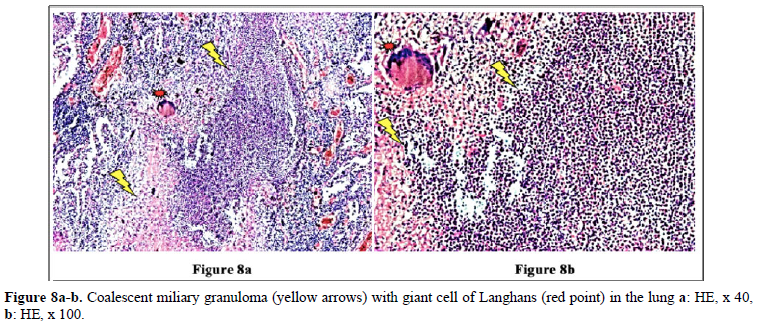








Many studies confirm that the incidence of TB is higher in RA than in the general population [2–6].
The prevalence and mortality of latent TB was also higher in our autopsy population with RA compared to the general population of Hungary [14–16]. Data of the U.S. Department of Health, Tuberculosis Control Division supported this statement as well [17]. Lowell’s [17] data roughly refers to the 4th quarter of the 19th century and are comparable to our data of RA patients who died between 1969-1992. The prevalence and mortality of TB is much higher in autopsy population compared to the general population [18]. The risk of encountering TB is reduced with decreasing incidence in the general population; however, it still can be found at autopsy [19].
Histopathology remains one of the most important methods for diagnosing tuberculosis [20]. The histology of tuberculosis is quite characteristic but stage dependent, therefore it is not always easy to make a correct diagnosis e.g. it may be problematic to distinguish fibrocaseous necrotic foci of TB in the lungs from rheumatoid nodules. The diagnosis of latent TB in RA is a great challenge for the rheumatologist mainly due to the limited response in elderly autoimmune patients. Despite the presence of TB, patients may have no clinical complaints or radiological abnormalities, and the value of the tuberculin skin test may be also limited due to inadequate or poor response of the patients [21 – 22], as well as the QuantiFERON blood test [23 – 24]. A positive Interferon-Gamma (γ) Release Assays (IGRA) result may not necessarily indicate TB infection with tuberculous mycobacteria [23]. A negative IGRA does not rule out active TB disease [24].
Histopathology remains one of the most important methods for diagnosing tuberculosis [20]. The histology of tuberculosis is quite characteristic but stage dependent, therefore it is not always easy to make a correct diagnosis e.g. it may be problematic to distinguish fibrocaseous necrotic foci of TB in the lungs from rheumatoid nodules. The diagnosis of latent TB in RA is a great challenge for the rheumatologist mainly due to the limited response in elderly autoimmune patients. Despite the presence of TB, patients may have no clinical complaints or radiological abnormalities, and the value of the tuberculin skin test may be also limited due to inadequate or poor response of the patients [21 – 22], as well as the QuantiFERON blood test [23 – 24]. A positive Interferon-Gamma (γ) Release Assays (IGRA) result may not necessarily indicate TB infection with tuberculous mycobacteria [23]. A negative IGRA does not rule out active TB disease [24].
Detailed medical history and targeted X-ray examination, as well as the tuberculin skin test, and the QuantiFERON blood test (despite their limitations) are key factors in the clinical diagnosis of latent TB with or without subclinical or atypical miliary exacerbation [25 – 27]. Microbiologic culture may be necessary, but takes time (may be critical premortem), the results are often false negative, and in clinically latent TB it appears not indicated. In our total autopsy population with RA (n=161) the proportion of female (n=116) to male (n=45) patients was: 2.57, and it was similar in RA patients with TB (n=21) as well; the rate of women (n=15) to men (n=6) was: 2.5. In Europa (including Hungary) more male (9647 persons) died of TB (rate: 63.8/100000), than females (3103 persons) (rate: 15.2/100000); the male: female ratio among of deceased people was 9647:3103 = 3.1089 [17]. In our autopsy population exclusively women (n=2 of 21) died of TB; the female: male ratio was 2:0 in contrast to to the European general population, where more males died of TB, than females [17]. According to our data women were more likely to be affected by TB in RA than in the European general population. The risk of active miliary dissemination (mTB) was particularly high in women; only females were involved by miliary dissemination of TB (all of 6 RA patients with mTB were women), the female: male ratio was 6:0) [18]. Miliary dissemination of tuberculosis (mTB) may be considered as a terminal phenomenon in our autopsy population, because of the limited numbers of granulomas involved only a few organs, except one patient with more extended dissemination (Table 5). The exudative (more serous, less cellular) and proliferative (more or less cellular) miliary foci without caseous necrosis or fibrous transformation and calcification also support the assumption that hematogenous dissemination was terminal i.e. premortem [18]. The exudative characters, beside proliferative epithelioid granulomas are consecutive stages of a basic pathological process, and in reverse order they may be regarded as histological evidence of an impaired and gradually decreasing immune reactivity [18]. The coalescent caseous cores of tuberculotic foci also support the poor reactivity of the patients; solid caseous necrosis or fibrous consolidated stages of miliary dissemination were not detected. The presences of exudative granulomas are unfavorable prognostic sign in elderly patients. The risk of death (probability of fatal outcome) in RA patients associated with fcTB or mTB is increased compared to the RA patients without TB, certified (proved, showed) by the significant and positive correlation between fcTB or mTB and mortality of TB. Consolidated anthracotic scars did not increase the risk of lethal outcome; the probabilitiy of death in RA patients with fTB was the same as that of RA patients without TB. The onset, and duration of RA did not influence the prevalence and mortality of inactive or active TB; inactive fTB or fcTB (n=15) and active mTB (n=6) developed at any time in the course of RA.
RA itself or its treatment modify the clinical symptoms of associated diseases and present atypical clinical manifestations leading to late recognition or missed diagnosis. The limited immune reactivity of elderly patients, the autoimmune character of RA, steroid and/or immunosuppressive drugs, and nowadays biological therapy may also play a role in missing the diagnosis of fcTB or mTb, including lethal cases. In our autopsy population only two cases of fTB were diagnosed; one of them was recognized by the patient’s detailed history, and the second one by the goal-oriented x-ray examination. The link between between clinical diagnosis and mortality of fTB or mTB was not significant, indicating the incidental nature of diagnosis. Clinical diagnosis of latent TB remains one of the main challenges of rheumatologists. In most cases the correlatios were not significant between TB (fTB, fcTB or mTB) and comorbidities (Ath, HT or DM), except fTB and mortality of Ath (c=0.58, c²=5.3478, p <0.0207) or fTB and prevalence of DM (c=0.56, c²=4.5372, p <0.0331) (Table 6). The lack of correlation between TB (fTB, fcTB or mTB) and comorbidities (Ath, HT or DM) indicate that these are sovereign phenomena (associated diseases) in RA, which may be present at the same time, and can lead to death independently from each other. This assumption is supported by the not significant “p” values, moreover by the negative values of association coefficients between them. The high values of association coefficient and the positive and significant correlation between fTB and mortality of Ath (c=0.58, c²=5.3478, p <0.0207) or fTB and DM (c=0.56, c²=4.5372, p <0.033) refer to a very close connection, but this does not necessarily mean a causal relationship; coincidence of parallel phenomena seems more likely. The strong positive correlation between fTB and mortality of Ath or fTB and prevalence of DM may be due to the equally high prevalence of fTB, fatal Ath or DM in aged RA patient cohorts. In our opinion fTB, Ath or DM are coincident comorbidities of elderly RA patients. This is supported by the fact that the mean age of RA patients with fTB, fatal Ath or DM was significantly higher, than the mean age of RA patients without fTB, Ath or DM: fTB: 70.92 years versus 64.87, p
CONCLUSION
The prevalence of post-primary TB was higher in RA autopsy patients compared to the general population of Hungary. Inactive fTB or fcTB (n=15) and active mTB (n=6) developed at any time in the course of RA.
The presence of fcTB or mTB increased the risk of mortality of RA patients, while consolidated anthracotic scars (fTB) did not. Women were more likely to be affected by TB in RA compared to the European general population. The risk of active miliary dissemination (mTB) and fatal outcome was particularly high in women; women with mTB died earlier then women without mTB. The onset, and duration of RA did not influence the prevalence and mortality of inactive or active TB.
- Bély M, Apáthy Á (2012) Clinical pathology of rheumatoid arthritis: Cause of death, lethal complications and associated diseases in rheumatoid arthritis.Academiai Kiadó 1-144.
- Carmona L, Hernández-García C, Vadillo C, Pato E, Balsa A, et al. (2003) Increased risk of tuberculosis in patients with rheumatoid arthritis. J Rheumatol 30: 1436-1439.
- He D, Bai F, Zhang S, Jiang T, Shen J, et al. (2013) High incidence of tuberculosis Infection in rheumatic diseases and impact for chemoprophylactic prevention of tuberculosis activation during biologics therapy. Clin Vaccine Immunol 20: 842-847.
- Liao TL, Lin CH, Shen GH, Chang CL, Lin CF, et al. (2015) Risk for mycobacterial disease among patients with rheumatoid arthritis, Taiwan, 2001–2011. Emerg Infect Dis 21: 1387–1395.
- Arkema EV, Jonsson J, Baecklund E, Rutting M, Bruchfeld J, et al. (2015) Are patients with rheumatoid arthritis still at an increased risk of tuberculosis and what is the role of biological treatment? Ann Rheum Dis 74:1212-1217.
- Jeong H, Baek SY, Kim SW, Eun YH, Kim IY (2017) Comorbidities of rheumatoid arthritis: Results from the Korean National Health and Nutrition Examination Survey. PloS One 12: e0176260.
- Saidane O, Sellami M, Mahmoud I, Tekaya BA, Ajlani H, et al. (2018) AB1054 Factors associated with tuberculosis in rheumatoid arthritis. Ann Rheum Dis 77: 1640.
- Ke WM, Chen LS, Parng IM, Chen WW, On AWF (2013) Risk of tuberculosis in rheumatoid arthritis patients on tumour necrosis factor-alpha inhibitor treatment in Taiwan. Int J Tuberc Lung Dis17:1590-1595.
- Lim CH, Chen HH, Chen YH, Chen DY, Huang WN, et al. (2017) The risk of tuberculosis disease in rheumatoid arthritis patients on biologics and targeted therapy: A 15-year real world experience in Taiwan. PloS One 12: e0178035.
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, et al. (1988) The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheumatol31: 315-324.
- Carson FL (1990) Mayer’s hematoxylin In Histotechnology, ASCP Press. pp: 100-103.
- Microbiology info.com (2018) Acid-Fast Stain- Principle, Procedure, Interpretation and Examples. Accessed on: June 12, 2018. Available online at: https://microbiologyinfo.com/acid-fast-stain-principle-procedure-interpretation-and-examples/
- Lentner C (1982) Statistical methods In Geigy scientific tables, 8th revised and enlarged ed: Ciba-Geigy Limited, Basle, Switzerland, Editor: Lentner C, Compiled by: Diem K, Seldrup J, volume 2, p: 227.
- World Health Organization (2020) Global Tuberculosis Report. Accessed on October 14, 2020. Available online at: https://www.who.int/publications/i/item/9789240013131
- Hutás I (1999) The history of tuberculosis in Hungary.” In: Tuberculosis past and present - Tuberculose: passé et present - [Editors: Pálfi Gy, Dutour O, Deák J, Hutás I] Golden Book Publisher Ltd., Szeged, pp: 37-42.
- Index Mundi. Tuberculosis mortality. Available online at: https://www.indexmundi.com/hungary/tuberculosis-mortality.html
- Lowell AM (1976) Tuberculosis in the world: trends in tuberculosis incidence, prevalence, and mortality at the beginning of the 3rd decade of the chemotherapeutic era. DHEW Publication, pp: 210-213.
- Bély M, Apáthy Á (2020) Prevalence and features of tuberculosis characterized by demographics, onset and disease duration of rheumatoid arthritis- A retrospective clinicopathologic study of 161 autopsy patients. EC Pulm Resp Med 9: 73-88.
- Flavin RJ, Gibbons N, O'Briain DS (2007) Mycobacterium tuberculosis at autopsy- exposure and protection: an old adversary revisited. J Clin Pathol 60: 487–491.
- Gupta M, Lobo FD, Adiga DSA, Gupta A (2016)A histomorphological pattern analysis of pulmonary tuberculosis in lung autopsy and surgically resected specimens. Patholog Res Int 1-7.
- Silva DGST, Silva BD, Torres PP, Santaana PJ, Junqueira-Kipnis AP, et al. (2012) Latent tuberculosis in rheumatoid arthritis: evaluating cellular response and high-resolution computed tomography. Arch Bronconeumol 48.5: 144-149.
- Ponce de León D, Acevedo-Vásquez E, Sánchez-Torres A, Cucho M, Alfaro J, et al. (2005) Attenuated response to purified protein derivative in patients with rheumatoid arthritis: study in a population with a high prevalence of tuberculosis. Ann Rheum Dis 64: 1360-1361.
- Slater M, Parsonnet J, Banaei N (2012) Investigation of false-positive results given by the QuantiFERON-TB Gold In-Tube assay. J Clin Microbiol 50: 3105-3107.
- Cho K, Cho E, Kwon S, Im S, Sohn I, et al. (2012) Factors Associated with Indeterminate and False Negative Results of QuantiFERON-TB Gold In-Tube Test in Active Tuberculosis. Tuberc Respir Dis 72: 416-25.
- Wolfe F, Michaud K, Anderson J, Urbansky K (2004) Tuberculosis infection in patients with rheumatoid arthritis and the effect of infliximab therapy. Arthritis Rheum 50: 372–379.
- Ponce de León D, Acevedo-Vásquez E, Alvizuri S, Gutierrez C, Cucho M, et al. (2008) Comparison of an interferon-gamma assay with tuberculin skin testing for detection of tuberculosis (TB) infection in patients with rheumatoid arthritis in a TB-endemic population. J Rheumatol 35: 776-781.
- Streeton JA, Desem N, Jones SL (1998) Sensitivity and specificity of a gamma interferon blood test for tuberculosis infection. Int J Tuberc Lung Dis 2: 443-450.
- Bély M, Apáthy Á (2019) b-cell related amyloidosis localized to the islets of Langerhans, type II diabetes mellitus and liponecrotic pancreatitis in rheumatoid arthritis: A postmortem clinicopathologic statistical study of 234 autopsy patients. Integr Diabetes Cardiovasc Dis 3: 94-110.
- Peter K, Zsuzsanna P, Zoltan K, Istvan W, Zsolt AT, et al. (2016) A 2-es típusú diabetes előfordulása és költségterheinek alakulása Magyarországon 2001–2014 között – az Országos Egészségbiztosítási Pénztár adatbázis-elemzésének eredményei. Diabetol Hung 24: 177–188.
- Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S (2016) Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res118: 535–546.
- Ruscitti, P, Cipriani P, Liakouli V, Iacono D, Pantano I, et al. (2019) Subclinical and clinical atherosclerosis in rheumatoid arthritis: results from the 3-year, multicentre, prospective, observational GIRRCS (Gruppo Italiano di Ricerca in Reumatologia Clinica e Sperimentale) study. Arthritis Res Ther 21.
- Agca R, Hopman LHGA, Laan KJC, van Halm VP, Peters MJL, et al. (2020) Cardiovascular Event Risk in Rheumatoid Arthritis Compared with Type 2 Diabetes: A 15-year Longitudinal Study. J Rheumatol 47: 316-324.
- Błyszczuk P, Szekanecz Z (2020) Pathogenesis of ischaemic and non-ischaemic heart diseases in rheumatoid arthritis. RMD Open 6: e001032.
- Dougados M, Soubrier M, Antunez A, Balint P, Balsa A, et al. (2014) Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA). Ann Rheum Dis 73: 62-68.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Cardiology and Diagnostics Research (ISSN:2639-4634)
- Oncology Clinics and Research (ISSN: 2643-055X)
- Journal of Renal Transplantation Science (ISSN:2640-0847)
- International Journal of Anaesthesia and Research (ISSN:2641-399X)
- Journal of Immunology Research and Therapy (ISSN:2472-727X)
- International Journal of Clinical Case Studies and Reports (ISSN:2641-5771)
- Dermatology Clinics and Research (ISSN:2380-5609)















