Research Article
Diabetic Nephropathy: From Diagnosis to Treatment
5037
Views & Citations4037
Likes & Shares
Diabetes is increasing worldwide and so is the Diabetic nephropathy (DN). Diabetes care poses a major challenge to both patients and also to the clinicians. However, the challenge is significantly increased in chronic kidney disease (CKD). Among patients with Type 1 and Type 2 Diabetes mellitus, DN remains the major cause for mortality and morbidity. Ongoing researches are increasing to identify the new biomarkers apart from albuminuria and glomerular filtration rate to predict and monitor the renal function among diabetic patients. Fortunately, it is preventable, if identified early and progression of the disease is also halted. Clinicians in primary care provides majority of the care, to the patients with diabetes, hence they are the ideal to recognize and to provide the initial treatment for CKD. This can be achieved through monitoring and controlling blood sugar levels and reducing blood pressure control using blockade of the renin-angiotensin system which prevents or slow down the progression of Diabetic nephropathy. Other methods to control and monitor blood pressure to manage renoprotection are under clinical investigation, which includes renal denervation, and endothelin receptor antagonism. It is highly recommended to evaluate eGFR and urine micro albumin to detect any deterioration in kidney function or for the presence of glomerular damage in CKD patients. With this clear understanding of the underlying mechanism of renal pathology, implicated in diabetic nephropathy, target specific Reno protective treatments are expected to become available over the next few years.
Key Points
-
After many decades DM still remains a major cause of ESRD (end-stage renal disease).
-
There is an increasing number of DM patients where glomerular filtration rate is reduced in the absence of albuminuria, with some classic morphological changes of diabetic nephropathy on renal biopsy.
-
For the early and accurate prediction, and monitoring of diabetic nephropathy, many clinical research activities have been increasing in number to identify the new biomarkers.
-
Many recent research evidences focused on drugs which inhibits renin - angiotensin system highlighted that early administration of such drugs among normoalbuminuric patients is not showing an effective result in preventing and developing of diabetic nephropathy.
INTRODUCTION
In the past decades, Diabetes mellitus and End Stage Renal Disease (ESRD) associated with diabetes becoming a major health challenge worldwide. Diabetes is the leading cause for Kidney failure, nearly 44% of the newly diagnosed diabetes patients were already with kidney problems [1]. Diabetic nephropathy (DN), a leading cause of end stage renal disease (ESRD) affecting approximately 20 - 30% of the patients with diabetes, it is also associated with the raising cardiovascular mortality [2]. The diabetic duration preceding the signs of diabetic kidney disease (DKD) and its progression is similar in both Type 1 and Type 2 diabetes (T2DM) [3]. Kidney involvement, directly and indirectly increases the involvement of other organs and increases morbidity and mortality in diabetic patients. The life expectancy of diabetic patients decreases when diabetic nephropathy is left untreated, thus prevention of this condition is of importance with early diagnosis and treatment. Clinicians in primary care provide majority of the care to the patients with diabetes, hence they are the ideal to recognize and to provide the initial treatment for Chronic Kidney Disease (CKD). Clinicians have to focus on the proper strategies to detect and to control kidney disease (CKD) among patients with diabetes. Micro albuminuria prevalence is associated with age, diastolic pressure, glycated hemoglobin, fasting plasma glucose and increases with duration of diabetes. There is a significant association between the blood pressure and insulin sensitivity about 60 - 80 % of the patients with diabetes Hypertension. There is an urgency to better identify patients with T2DM at early stages of CKD due to the exponential rise in prevalence of T2DM worldwide and the high risk of renal and cardiovascular complications in these patients.
In T2DM patients with uncontrolled glycaemia and elevated blood pressure, increase the risk of developing nephropathy. Factors such as duration of diabetes, glycemic control, age, elevated blood pressure and the presence of retinopathy are associated with the progression of diabetic nephropathy [4].
DEFINITION AND EPIDEMIOLOGY
Diabetic nephropathy is the leading cause of chronic kidney disease in patients starting renal replacement therapy [5] and is associated with increased cardiovascular mortality [6]. Diabetic nephropathy has been classically defined by the presence of proteinuria 0.5 g/24 h. The classical diabetic nephropathy is defined as the presence of proteinuria, which is also known as overt nephropathy, clinical nephropathy, proteinuria, or macro albuminuria. The study conducted in Europe showed that urine albumin in traceable quantity was not able to identified using the conventional method, perhaps it is predictable in development of proteinuria among patients with Type I [7-9] and type II diabetes [10], which is known as micro albuminuria or incipient nephropathy. European Diabetes (EURODIAB) Prospective Complications Study Group showed that the incident of micro albuminuria among T1DM patients were / was 12.6% over 7.3 years, and 33% in an 18-year follow-up study in Denmark [11]. UKPDS (U.K. Prospective Diabetes Study) study showed that among T2DM patients the micro albuminuria incident was 2.0% per year and the prevalence of micro albuminuria 10 years after diagnosis was 25% [12]. In T1DM patients, the occurrence of Proteinuria was 15 - 40% with the highest incidence was around 15 - 20 years of diabetes [13,14], whereas in T2DM the prevalence was highly variable ranges from 5 to 20% [15,12].
Diabetic nephropathy was prevalent among African Americans, Asians, and Native Americans than Caucasian population [16]. Nowadays the rate of increase of diabetic nephropathy was slowed down this might be due to the newer approach in the clinical practices and the early diagnostic methods to prevent diabetic nephropathy and the awareness among diabetic patients, thereby decreases the progression of established renal disease. Perhaps the practical possibilities of these measures were far below the expected goals [17]. Thus, the current review focused on the early screening tools, diagnostic methods and therapeutic strategies of diabetic nephropathy to increase reno and cardio protection among these high-risk groups, so as to reduce the incidence of diabetic nephropathy.
STAGES, CLINICAL FEATURES, AND CLINICAL COURSE
Diabetic nephropathy has been classified into micro albuminuria and macro albuminuria based on urinary albumin excretion (UAE). The current article provides the diagnosis, prognosis and treatment goals for Diabetic Nephropathy, which includes the heterogeneity of kidney disease among patients with diabetes, with special reference to T2DM and the classical paradigm of DN is not always observed in clinical practice. Perhaps there is an increasing usage of the term DKD (Diabetic Kidney disease) to refer persistent albuminuria and or reduced eGFR in diabetes set up and specific associated underlying renal pathology.
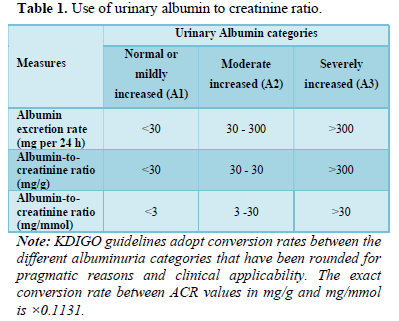
Kidney Disease Improving Global Outcomes (KDIGO) guidelines for CKD describes the use of three categories in terms of severity of albuminuria and the term micro albuminuria and macroalbuminuria is no longer be used in practice [18]. The KDIGO categories are summarized in the Table 1 it shows the use of urinary albumin to creatinine ratio (ACR) values of 3 to 30 mg/mmol or 30 to 300 mg/g (corresponding to micro-albuminuria) referred to as “moderately increased” (A2); and ACR values of >30 mg/mmol (>300 mg/g), corresponding to macro-albuminuria, referred to as “severely increased” (A3).

Clinical Features of DN
The distinct feature of well-established Diabetic Nephropathy is persistent albumin in urine with co-existing retinopathy, without any other kidney disease. In T1DM patients, the presence of these features showed the histological picture almost depicts the diabetic glomerulopathy [19]. In T1DM patients, 10 after the diagnosis the 10 years Diabetic nephropathy manifestation in T1DM patients in the initial 10 years after diagnosis was very rare, however, incidence was 35 per year between 10 - 20 years of diagnosis. On an average, approximately 15% of T1DM individuals were affected with severe (A3) albuminuria and 15% with moderate (A2) albuminuria [20]. The rate of incidence declines after 20 years of diagnosis, thus after 30 years of T1DM, the individuals with normal renal function and urinary albumin excretion, are at lower risk of developing DN [21]. Hence the development of diabetic nephropathy varies with individuals and it depends not only on duration of diabetes, but it also depends on other influencing factors such as blood pressure, Glycemic control and genetic susceptibility.
Globally, 90% of diabetic patients are T2DM, thus the majority of the individual developing DN, were T2DM [22]. When compared to T1DM, the epidemiology of DN, shows more variation in T2DM, with increased prevalence rate across the countries and the ethnic groups [20].
For instance, in a cross-sectional study conducted among 28 538 T2DM individuals without other kidney disease, were randomly screened from 33 counties, showed that albuminuria rate was higher in Asian and Hispanian groups (55 %) groups when compared to Caucasian groups (40.6%) [23]. There is likely to be the genetic component which clarifies the variation in these ethnic groups. This finding was well supported by the observational studies on familial clustering of Diabetic nephropathy and the genome wide association studies which identifies the genetic susceptibility loci [24]. There exists higher heterogeneity in clinical presentation of the T2Dm patients and the underlying pathological lesions of DKD. The variation between the time of diagnosis and the development of Diabetic nephropathy, where during diagnosis nearly 3% of the T2DM patients were already developed albuminuria. This might be due to the preceding undiagnosed diabetes and or pre diabetic condition [25], perhaps it can occasionally be an alternative renal pathology.
Moreover, Diabetic nephropathy occurs without co-existence of Diabetic retinopathy, this scenario is more common in T2DM when compared to that of T1DM [26,27]. In addition, presence of persistence albuminuria with retinopathy in T2DM shows that DN in majority of the cases [23], this is not the case always. Non diabetic form of kidney disease was observed in less than 10 percent of individuals with both diabetic nephropathy and diabetic retinopathy in a prospective biopsy study [26,28], perhaps the biopsy studies might introduce selection bias which might alter the findings [29]. These finding is not in line with the other studies which reported 100% specificity towards the combination of severe albuminuria and diabetic retinopathy in predicting classical histopathology of Diabetic nephropathy condition [23]. These variations in the finding reflect in the differences in epidemiology of diabetic nephropathy among T2DM population, however the small sample size, study design, biopsy practice might also contribute to the findings.
Histological Features of DN
In a minority of the cases kidney biopsy is used in diagnosis of diabetic nephropathy, however the typical histological features are explained in the international classification system. The histological features are classified as classes from I to IV based on the glomerular basement membrane thickening, mesangial expansion, nodular sclerosis (Kimmelstiel-Wilson lesion) and severe glomerulosclerosis, respectively. 30 Further to these classic glomerular features, interstitial fibrosis, interstitial fibrosis and tubular atrophy (IFTA), arteriosclerosis and arteriolar hyalinosis were also present very frequently.
Pathophysiology of Diabetes Nephropathy
Diabetes, Hypertension, cardiovascular disease, family history of kidney disease was considered as the important risk factors. Other risk factors include urinary tract infection, autoimmune disease, obesity, kidney damage, and systemic infection [31]. Early diagnosis and awareness of DKD, combined with reno-protective management practices, reduces diabetes related ESRD [32].
In diabetic patients, the major contributing factor for kidney disease is hyperglycemia, whereas other factors such as family history of nephropathy, hypertension, and increased duration of diabetes also plays a considerable role [33]. Protein kinase C (PKC) plays a major role in diabetic nephropathy, it consists of 12 isoforms and are involved in the progression and pathogenesis of diabetic nephropathy [34]. Hyperglycemia activates PKC and also activates other stimuli such as reactive oxygen species, AGE receptors, AGEs (Advanced glycation end products) and angiotensin II in diabetic nephropathy condition [35].
Determining etiology is important, when evaluating in patients with diabetes. In diabetic patients, CKD might be due to diabetes, in the presence of retinopathy and albuminuria and causes unrelated to diabetes are negative [36]. Progression of the disease occurs with inadequate or lack of appropriate care for diabetes and CKD and the scope of prevention is also lost when both of these conditions are undiagnosed [37]. Estimated glomerular filtration rate (eGFR) (2) and increased levels of urine albumin (>30 mg/g) is the two classical guideline-endorsed biomarkers for the classification of CKD [38]. Albuminuria and eGFR is a strong predictor for the progression of the renal disease and CVD, however the continuous search for the novel biomarker helps to identify the high-risk patients at the early stages. Biomarkers improve the risk stratification and also help in better understanding of the pathophysiology of the renal disease which leads to novel therapeutic targets.
T2DM is a multifactorial disease with various pathogenic molecular processes and heterogeneous histopathological structure [39], thus multiple biomarkers capture the different pathogenic process of renal damage, might provide more clear insight of the patient’s actual pathological condition. Nearly 30 % of the T2DM patients with kidney disease also have other renal disease without diabetic nephropathy [40]. Hypertension is the well-established risk factor for the progression of the DKD and it is common among patients with diabetes. ADVANCE trail highlighted that the systolic blood pressure is independently associated with the renal outcome [14]. Beyond reducing blood pressure, renin angiotensin aldosterone system inhibitors (RAASi) show a Reno protective effect [41-45].
Clinical Progression of Diabetic Nephropathy
In the early stages of DN, blood pressure is normal with no evident micro albuminuria, but with high eGFR. This indicated that the temporary enhanced filtration or hyper filtration. The next stage of DN is characterized by micro albuminuria which correlates with glomerular mesangial expansion where GFR come back to the normal range and increase in blood pressure. In the final stage, macro albuminuria accompanies with an increasing in blood pressure and declining GFR which correlates with histological appearance of the glomerulosclerosis and Kimmelstiel-Wilson nodules [46].
The risk factors for progress of diabetic nephropathy in Indian population were systolic blood pressure, HbA1c, age, duration of diabetes, poor Glycemic control and presence of retinopathy [4]. The presence of diabetic retinopathy with normoalbuminuria is significantly associated with the rate of development of macro albuminuria [4]. Diabetic retinopathy was present in all proteinuric T2DM patients, when compared to only 24% of non proteinuric T2DM counterparts [47].
Moderately Increased Albuminuria (A2)
The onset of moderately increased albuminuria predicts the onset of established diabetic nephropathy among patients with diabetes, this relationship has been reported in various studies [7-11]. Further moderately increased albuminuria indicates the increased cardiovascular risk in both T1DM and T2DM patients. [48,49]. In a study conducted among 286 T1DM patients between 1979 and 1984 were followed up prospectively for 18 years [11]. Of 286 patients 79 were developed moderately increased albuminuria (A2), 27 (34%) subsequently progressed to severe (A3) albuminuria. The study findings revealed that even though the sudden decrease to normoalbuminuria occurs (in the absence of RAAS inhibitors), it was rare and this decrease doesn’t occur once severe albuminuria (A3) has developed. Other observational and interventional studies have highlighted the same findings in T2DM patients [50].
The HOPE trial showed that the individuals without baseline micro albuminuria developed overt nephropathy [51], this observation concluded that moderated albuminuria showed first clinically detectable stage of diabetic nephropathy, without intervention it will progress to advance stage and less reversible stages of kidney disease in a significant proportion of affected individuals. Further the histological lesions of diabetic glomerulopathy were developed, when moderate albuminuria was detected, and in some cases even before the clinical manifestation histological lesions were developed [52].
Perhaps other study findings suggested that the traditional paradigm of an inevitable progression of albumin from moderate to severe and fall in eGFR which is not observed in all patients with diabetic nephropathy. Specifically, the rate of regression of albuminuria is greater in both T1DM and T2DM individuals. In 6 years follow up study on regression of albuminuria in 386 T1DM patients with moderate albuminuria [53], showed the cumulative incidence of progression of albuminuria from moderate to severe was 19%. In the same period of time 59% of individuals were regressed from moderate to normoalbuminuria, it was an observation which cannot be explained by the RAAS inhibition. The regression of moderate to normoalbuminuria among T2DM patients occurs in 23.6% [54]. Moderately increased albuminuria (A2). As well as indicating increased cardiovascular risk in both T1DM and T2DM, 20,21 the traditional paradigm is that the onset of moderately increased albuminuria (A2), previously termed microalbuminuria, predicts the onset of established DN. 22-25 number of studies have reported this relationship in both T1DM and T2DM. Hovind [11] recruited 286 people with T1DM between 1979 and 1984 who were followed prospectively for a median of 18 years. 25 Of the 79 who developed moderately increased (A2) albuminuria, 27 (34%) subsequently progressed to severe (A3) albuminuria. The authors reported that although spontaneous regression to normal albumin excretion did occur (in the absence of RAAS inhibitors), it was rare and was not observed once severe (A3) albuminuria had developed. Similar results are seen in a number of other observational studies and interventional trials, as well as in T2DM. 26 In the HOPE trial, in which participants at increased cardiovascular risk were randomized to ramipril or placebo, moderate albuminuria (A2) at baseline was present in 31.8% of the 3577 people with T2DM. 27 After 4.5 years, 225 (20%) participants with and 41 (2%) without baseline microalbuminuria developed overt nephropathy (relative risk [RR] 14, 95% CI 10-19, P <.001). These observations led to the conclusion that in many cases, moderate (A2) albuminuria represents the first clinically detectable stage of DN, which without intervention will progress to more advanced and less reversible stages of kidney disease in a significant proportion of affected individuals. This is also supported by the development of histological lesions of diabetic glomerulopathy by the time that moderate albuminuria is detected (and sometimes even before any clinical manifestations are apparent). 28 However, other data challenge this concept and suggest that the traditional paradigm of an inexorable progression from moderate albuminuria through severe albuminuria to progressive fall in eGFR is not seen in all people with DN. In particular, the rates of regression of albuminuria may be greater than previously appreciated in both T1DM and T2DM. Perkins et al reported regression of albuminuria in 386 people with T1DM and moderate (A2) albuminuria who were evaluated over a 6-year follow-up period. 5 After 6 years, the cumulative incidence of progression to severe (A3) albuminuria was 19% (95% CI 14-23%). Over the same time period, the cumulative proportion of those that regressed to normo-albuminuria was 59% (95% CI 54-64%), an observation that was not explained by RAAS inhibition. In a separate study, regression from moderate (A2) albuminuria to normo-albuminuria in a cohort of T2DM was reported to occur in 23.6% of cases. 29 Methodological aspects of assessing albuminuria.
Methodological Aspects of Assessing Albuminuria
Apart from various clinical trajectories, the albuminuria assessment was more complex due to intra individual variation in albumin excretion. Three separate urine samples were collected from a cohort of proteinuric CKD patients, showed 29.7% variation for ACR in random samples and 32.5 % variation in early morning samples (55). The same kind of variation was observed in the measurement of urine albumin excretion rate, where the challenge was to accurate collection of 24 h urine samples [56]. Due to these above said inconveniences in albumin excretion measurement, ACR is the preferred method to access albuminuria in clinical practice. Majority of the guidelines such as ADA (American Diabetes Association), NICE (the National Institute for Health and Care Excellence), and EADS (European Association for the Study of Diabetes), recommended annual screening of ACR to detect moderate albuminuria among patients with diabetes, with prerequisite of repeated testing of elevated findings [57-59]. It is mandatory to consider the biological variations in values of ACR when monitoring the continuous changes / response to particular treatment and interpreting the changes and examining serial trends is a more reliable approach.
The clinicians should be aware of the condition where there is an elevated levels of albuminuria and the risk of misinterpreted diagnosis, which includes severe hyperglycemia heart failure, menstruation active systemic infection/inflammation, and heavy exercise in the preceding 12 to 24 h and urinary tract infection. Further it is difficult to interpret urinary ACR in patients with long term urinary caterers. Urine dipstick testing is not recommended for quantifying and monitoring the degree of albuminuria over the time [60].
Non-albuminuric DKD
The decreased eGFR occurs in normal urinary albumin excretion in T1DM and T2Dm individuals [61,62]. Generally non-proteinuric chronic kidney disease frequently points towards the etiologies that are ischemic in nature or predominant tubulo-interstitial pathologies [63,64]. Perhaps the non proteinuric diabetic nephropathy has been described along with the typical histopathological changes in diabetic glomerulopathy [65,66]. Typical pathological findings have been identified from 526 renal biopsies from patients with eGFR less than 60 mL/min/1.73 m2 retrospectively [66]. Among 528 renal biopsies, 16.7 % had non proteinuric diabetic nephropathy and 83.3 % has proteinuric diabetic nephropathy. In the overt proteinuria group 3.6 % had normoalbuminuria and 13.1% had moderately increased albuminuria.
However, as seen in other CKD forms, the degree of proteinuria is a strong predictor of progression risk and that non-proteinuric DN has a better prognosis [67]. Non proteinuric diabetic nephropathy showed lowered blood pressure and lesser pathological lesion. Further, the non proteinuric group showed 86.6 % better 5-year CKD progression free survival when compared to the proteinuric group (30.3%) [66].
Many empirical studies highlighted the reduced risk of CKD progression and or the development of ESRD in non albuminuric DKD versus diabetic nephropathy with significant albuminuria [68,69]. Perhaps this should interfere with the development of non albuminuric DKD risk factors mortality and majority of the cardiovascular events when compared to the individuals without kidney disease, however there is a greater risk in the presence of albuminuria [70]. There are many underlying possibilities which might explains the occurrence of non proteinuric diabetic nephropathy, which includes co existing condition of other vascular disease, tubulo intestinal fibrosis, decline in eGFR due to previous episodes of AKI, decreased albuminuria due to RAAS inhibitors [71].
Diagnosis
In majority of the patients at early stages of CKD renal function declines at a very slow phase and the rate of decline varies from individual to individual. This is due to the fact that in CKD stage 1 to stage 3 progression of the disease is asymptomatic and early detection requires laboratory testing.
- Blood pressure control slows the progression of kidney and cardiovascular disease. During each hospital visit blood pressure has to be monitored. The target BP is 130/80 mmHg. High systolic blood pressure of greater than 180 mmHg has to be lowered very slowly, further lowering BP delays the micro to macro albuminuric progression and slows the progression to renal failure.
- Elevated levels of urine IgG and NAG are early indicators for renal damage associated with increased proteinuria and serum ACE. Therefore, measurement of urinary IgG and NAG can be considered even before onset of micro albuminuria, as a significant marker for diabetic nephropathy among patients with T2DM. It is also helpful to identify the patients who are at high risk of diabetic nephropathy [72,73].
- Urinary albumin: Proteinuria develops in T2DM after 5 to 10 years of onset of diabetes, thus regular screening of urinary albumin is mandatory.
- A recent biomarker LFABP has been shown to be positively correlated with declining renal function in Indian population [72,73].
- In clinical management regular estimation of glycated hemoglobin and screening of micro albuminuria helps to prevent diabetes complications such as diabetic nephropathy [74].
Clinical Approach in Diagnosis of DKD
Diabetes kidney disease is the clinical diagnosis in majority of the cases; however, kidney biopsy was the gold stand procedure for the diagnosis and prognostic treatment, but the biopsies was performed only when suspecting any other alternative pathology.
Screening
In general, DKD doesn’t show any symptoms, thus ADA and KDIGO guideline recommended that all individuals with T2DM should undergo the renal function test and albuminuria measurement at the time of diagnosis and annually thereafter. In T1DM patients the renal function test and albuminuria measurement should be started from five years after diagnosis of T1DM [57,75]. Albuminuria was accessed best using ACR in spot urine samples, preferably in the early morning samples. 24 h urine collection or timed collection was also used to measure the albumin excretion was also appropriate, perhaps it is less convenient and prone to collection errors. Renal function test should be assessed using a serum-creatinine based calculation (CKD-EPI equation recommended due to its superior performance in the eGFR range 60-90 mL/min/1.73 m2). Increased Serum creatinine, reduced eGFR levels and the presence of macroalbuminuria were significantly associated with increased risk of progression of sight-threatening diabetic retinopathy (STDR) [76].
In general, all T2DM individuals with DKD show some degree of DR. The sudden onset or the rapid progress of proteinuria, absence of diabetic retinopathy and presence of hematuria and urine sedimentation showed non-diabetic etiology and recommended a kidney biopsy. This condition is also known as normoalbuminuric DKD where presence of CKD in the absence of albuminuria [77]. Thus, it is necessary to keenly watch the kidney function in T2DM individuals with both estimation of albumin in the urine and estimation of eGFR. Further it is shown that in Indian population the CKD Epi [Sr Cr] formula can be used to determine the eGFR [78,79]. If a reduction in eGFR or an increase in albuminuria is detected, this should be confirmed on repeat testing over 3 to 6 months; a minimum of two elevated ACR levels more than 3 months apart are required before an individual is considered to have increased albuminuria [32]. This is to differentiate from transient changes as well as to account for the intra-individual variation that is seen in ACR. Similarly, two eGFR values below 60 mL/min/1.73 m2 at least 90 days apart are required to make a diagnosis of CKD
Confirmation of Persistent Abnormalities
If there is an increasing trend in albuminuria and there is a reduction in eGFR was detected, this should be confirmed by repeating the test over 3 to 6 months’ time period. The minimum of two elevated levels of ACR for more than 3 months apart are required before confirming that the individuals are considered to have increased albuminuria [80]. This confirmation is to identify the individuals from the transient stage and also allows to detect the intra individual variation which is seen in ACR levels. Likewise, the two eGFR values below 60 mL/min/1.73 m2 at least 90 days apart are required to make a diagnosis of CKD.
Clinical Diagnosis of DKD
The clinical diagnosis of DKD can be confirmed when there exist a persistent moderate (A2) and severe (A3) albuminuria and or a persistent declining in eGFR to 2 in 5 years after the diagnosis of diabetes among T1DM individuals. Nearly in 95 percent of individuals, diabetic retinopathy will also be present [19], without any other clinical suggestions of alternative kidney disease. Albuminuria is not necessary be required for the diagnosis of DKD in cases of persistent declining in eGFR, however this scenario also be considered in other forms of non-albuminuric kidney disease and albuminuria in the absence of diabetic retinopathy in T1DM patients.
The clinical diagnosis of DKD will be more challenging in T2DM patients as there is an increasing heterogeneity in the clinical presentation, though the same principle of persistent albuminuria and declining in eGFR applies. Presence of albuminuria is not mandatory in DKD diagnosis provided there is a declining eGFR 2. Prolonged duration of diabetes and presence of diabetic retinopathy are the key pointers for the diagnosis, however shorter duration of diabetes and/or the absence of retinopathy were useful to rule out DKD among individuals with T2DM. Thus, it is important to evaluate the key features which might indicate the alternative forms of kidney disease and proceed with renal biopsy when there is a need for diagnostic uncertainty.
Evaluation
- There is a positive correlation between urine albumin excretion rate and estimated glomerular filtration rate (eGFR2) indicating that these two parameters provide a complimentary benefit in management of chronic kidney disorder [81].
- A simple inexpensive screening procedure for urinary protein excretion which can be used as a diagnostic test even in the outpatient clinic has been reported in the Indian population. Estimated proteinuria (EPE) is useful in serial evaluation of kidney function [82]. Features that may indicate alternative forms of kidney disease Features that may indicate alternative forms of kidney disease Clinical approach to diagnosis of DKD In many cases, DKD is a clinical diagnosis. A kidney biopsy is the gold standard test for diagnostic and prognostic information, but in most centers is usually only performed when an alternative renal pathology is suspected.
Features that may Indicate Alternative Forms of Kidney Disease
Non-diabetic forms of kidney disease may be suggested by the following:
- Unusual trajectory of decline in eGFR / onset of albuminuria. The eGFR declines rapidly or sudden increase of albuminuria are not the usual typical diabetic nephropathy, severe albuminuria in the first five years of diagnosis among patients with T1DM. By focusing on the trend of eGFR will ease in identifying the previous episodes of AKI, these can greatly be recognized to be associated with onset of CKD and its progression [83-85].
- Nephrotic syndrome / severe albuminuria (ACR > 300 mg/mmol or > 3000 mg/g): Even though diabetic nephropathy is a known cause of nephrotic syndrome, primary glomerular disease is also observed, specifically during the acute onset of nephrotic syndrome.
- Urinary sediment: The invisible hematuria is not the typical finding in diabetic nephropathy, but it can occur. The presence of hematuria in urinalysis is not specifically helpful and also lacks the ability to distinguish between diabetic and non-diabetic kidney disease [86]. Perhaps, the presence of red call caste during urine microscopy, signifies that the presence of alternative pathology specifically a glomerulonephritis.
- The clinical features which are suspicious for other systemic diseases which commonly cause the kidney diseases such as HIV, connective tissue disorders.
- Family history of non-diabetic forms of kidney disease.
Differential diagnoses to consider in the setting of non-albuminuric DKD
The early evaluation for non albuminuric diabetic nephropathy should include the following:
- Earlier episodes of AKI: Ischemic nephropathy: hypertension, history of smoking, aortic disease or asymmetric kidneys on renal ultrasound. This scenario is sometimes termed under a common term diabetes kidney disease in cases of without renal biopsy and many other risk factors of ischemic nephropathy and are common among individuals with diabetes. Renovascular disease can also be suggested by large (>30%) declines in eGFR after initiation of RAAS inhibitors.
- Dysproteinemia-related renal disease: There are many renal diseases which are associated with the dysproteinemias, which has been screened with the help of serum electrophoresis and assay of serum free light chains [87].
- TIN (Tubulointerstitial nephritis): It is traditionally associated with the eosinophilia and urinary leukocytes, perhaps present with the normal urinary sediment. TIN might be due to the other medications used by the patients such as diuretics, proton-pump inhibitors, antibiotics, non- steroidal anti-inflammatory drugs and a careful medication history to establish temporal links between initiation of culprit medications and onset of eGFR decline can be useful. Diagnosis requires kidney biopsy.
Prognosis
T2DM individuals with renal disease are at increased risk of progression of CKD, cardiovascular disease, ESRD and mortality [48,88]. These statements are undoubted, there are certain major methodological considerations relating to the underlying data. This data includes the ethnicity, clinical variability (such as Glycemic control, nephron endowment), on rate of CKD progression, hence the variation in the baseline characteristic of the study group might lead to the variation in the reported outcome rates. As a result of new effective intervention which slows downs the progression of Diabetic nephropathy, or alters the cardiovascular risks, along with the upgraded guideline which supports more aggressive approach in the management (lowering of cholesterol and blood pressure) have been impacted upon prognosis over the time. There exists a sustainable improvement in the patient outcome in the past four decades along with various clinical course of diabetic nephropathy, for instance the there is an increasing recognition of regression of albuminuria and consistency of eGFR among diabetic individuals with diabetic nephropathy [89,90].
In T1DM patients, diabetic nephropathy would be detected in early stages in the natural history of the condition where as it is not the case in T2DM individuals where it is difficult to compare the natural history of Diabetic kidney disease among these two groups, further there exist and visible difference in age and baseline comorbidity among these groups. The eGFR estimation and measurement, albuminuria assessment plays a major role in the assessment in CKD progression [48,91-94].
Among T1DM patients, many study findings suggested that the progression and development of renal impairment is the major underlying cause for mortality. The Finn Diane (Finnish Diabetic Nephropathy), study group showed the mortality rate among 4201 T1DM cohort over a 7-year period of time and mortality was increased among patients who developed diabetic kidney disease [49]. Further there exists a relationship between the severity of renal disease and outcomes. There exists no difference between general population and normoalbuminuric patients, perhaps moderate and severe albuminuria and end stage renal disease showed exponential increase in standardized mortality ratio. These findings were similar to data of many studies, further suggesting that excess mortality in T1DM was more predominant among patients who developed chronic kidney disease, specifically who progressed to ESRD [13]. This kind of high relative risk of T1DM individuals who develops nephropathy and who doesn’t develop nephropathy are highly noticeable and reflects the younger age of T1Dm cohorts and relatively low event rate in patients without kidney disease.
CKD prognosis consortium conducted a meta-analysis and compared the level of eGFR and albuminuria levels among individuals with and without diabetes, the data were pooled from large cohort studies, that together formed millions of participants (nearly 13 % had diabetes specifically T2DM) [48]. As estimated, CV mortality, ESRD rate and mortality were higher with increasing albuminuria and decreasing eGFR values. In general diabetes is associated with mortality and morbidity, the mortality risk was similar among individuals with and without diabetes at a fixed albumin and eGFR reference point. To put in another way, the absolute risk of Cardiovascular mortality, ESRD, and mortality rate was high among CKD patients with diabetes when compared to their counterparts (i.e., without diabetes), perhaps the relative risk of these outcomes is alike throughout the range of eGFR and albumin level, which highlighted the importance of development of CKD upon increasing the risk of adverse outcomes. The risk of cardiovascular mortality and risk of mortality was considerably higher than the risk of progression of ESRD among T2DM patients, although the relative risk with DN and without DN was lesser than in T1DM individuals this is due to the rate of event without kidney disease [12].
There exists a wide difference in the rate of progression of CKD in diabetic kidney disease with reference to the trajectory of eGFR and progression rate of ESRD. In DCCT trial (Diabetes Control Complications Trial) the extended follow up of the participants showed that the change in eGFR of -1.37 mL/min/1.73 m2/year among T1DM patients for whom the mean duration of DM at the time of baseline was 5.9 years with normal albumin and eGFR levels [95]. The rate of decline in eGFR was more rapid at -5.4 mL/min/1.73 m2/year, after the onset of severe albuminuria [96], perhaps inter individual variation also existed within this range. In the subsequent 10 years of follow up, nearly 32 % of individuals belongs to severe albuminuria still had an eGFR >60 mL/min/1.73 m2, while 16% of individuals progressed to ESRD the latter equating to an incidence rate of ESKD of 1.4 events/100 person years.
The incidence of ESRD is between 2.2 to 4.1 event/100 individuals/ year was shown in a combined analysis of 4 cohort studies, which includes 1518 individuals with T1DM and diabetic nephropathy [97]. In T2DM individuals, discrepancies exist in the rate of progression of CKD and ESRD incidents. In the observational prospective study conducted among 227 Caucasian T2DM individuals with nephropathy for an average of 6.5 years (3 years follow up period), in whom GFR was measured annually with Chromium EDTA clearance highlighted several modifiable risk factors of enhanced progression in kidney disease and increased mortality [98]. In a randomized control IDNT trial (Irbesartan Diabetic Nephropathy Trial) T2DM individuals with decreased eGFR and severe albuminuria were treated with irbesartan, amlodipine / placebo showed - 5.5 mL/min/1.73 m2/year in creatinine clearance using irbesartan [45]. Likewise, the average change in eGFR of - 4.4 mL/min/1.73 m2/year was observed in RENAAL trial (Reduction of End Points in NIDDM with the Angiotensin II Receptor Antagonist Losartan trail) [44].
Discrepancy in ESRD Rate
Patients with chronic kidney disease and severely decreased glomerular filtration rate (GFR) are at high risk for kidney failure, cardiovascular disease (CVD) and death. In a study conducted among community health setting included more than 42000 individuals with diabetes showed that 20.2% had estimated GFR 2, 18% had albuminuria and 6.2 % had both albuminuria and declining eGFR [99]. The progression rate of ESRD varies from 0.02 to 22 events/100 person/ years, with eGFR at the baseline and progression rate. The risk of mortality was based on the eGFR and albuminuria, further it is the major determinant for survival. The clinical characteristics associated with the increased risk of progression of CKD includes rate of decline in eGFR, serum uric acid, HbA1c, Diabetes duration, severity of albuminuria, family history and coexisting micro vascular complication [100].
Many tools have been developed to help clinicians to estimate the progression of ESRD among patients with CKD. The existing Kidney Failure Risk Equation (KFRE) includes only four variables such as age, gender, eGFR and UACR to predict 5-year risk of ESRD. This equation was validated externally in a large cohort of 721,357 from 31 cohort studies and the findings highlighted that this equation is equally performing well in people with and without diabetes [101,102]. There exist few tools which predict the onset of kidney failure among patients ESRD, which is the most expensive outcome of CKD. There exist other prediction tools, perhaps they have not undergone a robust validation [103-106]. Further these tools were not developed to evaluate individuals with G4 CKD stage or predict other potential common events which includes pre KRT and non-fatal CVD events. In the advanced stage of G4 CKD individuals, the risk prediction tool was developed which includes diagnosis of diabetes and concurrently predicted the 4-year risks of cardiovascular events, ESRD or mortality [107].
Prevention of Diabetic Kidney Disease
Early diagnosis and detection of risk factors for diabetic complications such as nephropathy, neuropathy and cardiovascular complications is a major step in prevention of CRS. Hypertension is the major factor which accelerates the progression of Diabetic renal disease. Early diagnosis of microalbuminuria is the key factor to detect nephropathy. Hyperglycemia is considered as the major determinant factor for the progression of diabetic complications especially diabetic nephropathy. Hyperglycemia sets a series of metabolic abnormalities and hemodynamic which lead to histological and clinical changes in diabetic nephropathy. Various studies highlighted that the intensive glycemic control slowdown the rate of progress of micro and macro albuminuria in diabetic patients [108]. Glycemic control prevents or delays the diabetic related complications. Thus, self-management of blood sugars with diet, exercise, medication, monitoring daily blood glucose level and education helps in controlling blood sugar.
Management of Diabetic Kidney Disease
Progression of micro albuminuria and macroalbuminuria can be prevented by tight control of blood pressure (target ≤ 130/80 mmHg) and intensified dietary management. Intense blood glucose monitoring and maintaining HbA1c significantly reduces the risk of diabetic nephropathy [82]. In patients with diabetic nephropathy, either ACE inhibitor or ARB is the first line of treatment, combination of these two is no longer recommended. Monitor ACR, eGFR and serum potassium. In patients with proteinuria, limiting protein intake to 1 g/kg daily is recommended. A simple inexpensive screening procedure for urinary protein excretion which can be used as a diagnostic test even in the outpatient clinic has been reported in the Indian population. Estimated proteinuria (EPE) is useful in serial evaluation of kidney function [82]. The risk factors for progress of diabetic nephropathy in Indian population were systolic blood pressure, HbA1c, age, duration of diabetes and presence of retinopathy [4].
Nearly less than 20% of the patients with moderate to severe kidney disease are aware about their condition [109]. The diabetes educators’ role in education of preventive and management strategies for DKD is also influential to optimize outcomes. Diabetic educators should focus on awareness and education on diabetes and its complication, self-management of diabetes, management of kidney disease along with other co morbid conditions. Diabetic education would assist the diabetic patients to understand their disease and help the patients to cope up with the co morbid conditions and its complexities.
Management
Individuals with chronic kidney disease should be managed as follows:
- Using ARBs and ACE-inhibitors in patients with Micro and macro albuminuria have to be titrated to maximum tolerance test.
- Controlling the blood pressure slows down the progress of micro and macro vascular complication. Intensified dietary management (reduce the Salt intake) and blood pressure management (target ≤ 130/80 mmHg).
- Intensify management of blood glucose, Maintaining the HbA1c less than or equal to 7% significantly reduces the risk of nephropathy and progression of microvascular complication [82].
- In patients with diabetic nephropathy, either ACE inhibitor or ARB is the first line of treatment, combination of these two is no longer recommended.
- Monitor ACR, eGFR and serum potassium
- Advise limiting protein intake to 1 g/kg daily if proteinuric
- Intensification of other cardiovascular and renal protection measures.
- In patients with diabetic nephropathy, use of phosphate binders (sevelamer hydrochloride) is recommended to decrease hypophosphatemia commonly observed in this patient group [110].
- The risk factors for progress of diabetic nephropathy in Indian population were systolic blood pressure, HbA1c, age, duration of diabetes and presence of retinopathy [4].
- Given that vit-D deficiency can have significant impact on albuminuria, supplementation with calcitriol should be considered in these patients as it has been shown to provide beneficial effects on micro albuminuria and therefore diabetic nephropathy [4].
Metformin Recommendation
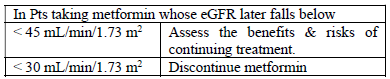
- Recommending Metformin to patients with eGFR less than 30 mL/min/1.73 m2 is contraindicated.
- It is not recommended to start metformin in patients whose eGFR falls in between 30 - 45mL/min/1.73 m2 Patients on metformin have to check eGFR at least once in a year.
- Frequent renal function assessment is necessary for the patients who were at risk of developing renal impairment.
EVALUATION OF PATIENTS WITH DIABETIC NEPHROPATHY
Prevention and Treatment
The basic fundamentals for the prevention of diabetic nephropathy are the treatment regimens of its well-known risk factors such as dyslipidemia, hypertension, smoking and hyperglycemia, which are also risk factors for cardiovascular disease and should be controlled intensively.
Intensive blood glucose control
Clinical trials have consistently emphasized that HbA1c level less than 7 % is associated with the reduced risk factors diabetic nephropathy in both T1DMand T2DM individuals. The DCCT trial (Diabetes Control and Complications Trial) highlighted that the intensive diabetic treatment decreases the incidence of micro albuminuria (36%). Further it is noted that individuals followed strict glycemic control showed a long-lasting reduction of approximately 40% in the risk of developing micro albuminuria and hypertension even after 7 to 7 years at the end of DCCT trial [111]. The risk of developing micro albuminuria was reduced by 30% in the intensively treated group for hyperglycemia was showed in UKPDS study [112]. Further Kumamoto Study highlighted that intensive hyperglycemic control reduces the risk of developing micro and macro albuminuria [113]. Thus, the intensive glycemic treatment should focus to attain A1c <7% should be achieved as early as possible to prevent micro albuminuria.
Intensive blood pressure control
The treatment regimens of hypertension significantly reduce the risk of cardiovascular risk and microvascular events in patients with diabetes. Hypertension is the common phenomenon in patients with diabetes even in the absence of renal involvement. Nearly 70% of T2Dm patients and 40% of T1Dm individuals with normoalbuminuria showed blood pressure of 140/90 mmHg [114]. The UKPDS study showed gradual reduction of systolic blood pressure from 154 to 144 mmHg which reduces the risk of developing micro albuminuria by 29% [115]. Hypertension Optimal Treatment study highlighted that the risk of cardiovascular events was reduced by 50% in patients with diabetes, when diastolic blood pressure is reduced by 85 to 81 mmHg, which is not observed in non-diabetic individuals. The target BP should be lower (130/80) in patients with diabetes than their counterparts [116].
Renin-angiotensin system blockade:
The key role of ACE inhibitors in diabetic nephropathy prevention is not well defined in T1DM individuals. In normotensive normoalbuminuric T1Dm individuals the used of perindopril for the period of 3 years delays the increases of albuminuria [117]. In T2Dm individual both ARBs and ACE inhibitors reduce the risk of diabetic nephropathy and incidence of cardiovascular events [118,51]. The MICRO-HOPE (Heart Outcomes Prevention Evaluation) study showed that the risk of overt nephropathy was reduced by 24% and cardiovascular mortality by 37% among T2DM individuals aged 55 years with one cardiovascular risk by using ramipril (10 mg/day). Further ramipril decreases UAE at the end of the study period [51]. Thus, the use of ACE inhibitors showed reno and cardio protection among individuals with T2DM.
Treatment: Micro and Macroalbuminuric patients:
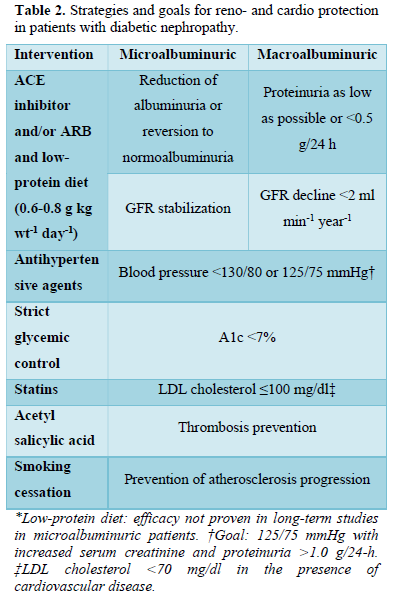
The main aim in the treatment plan is to prevent the progression of microalbuminuria to macroalbuminuria, prevent / reduce the declining renal function in macroalbuminuric patients and cardiovascular events. The treatment plans are as same as the prevention of diabetic nephropathy, perhaps in treatment the multiple and intensive strategies must be implemented. The strategies and goals are described in Table 2.
Intensive blood glucose control:
The effect of strict glycemic control on the progression from micro- to macroalbuminuria and on the rate of renal function decline in macroalbuminuric patients is still controversial. The DCCT study highlighted that the T1DM individuals with microalbuminuria, who intensified glycemic control close to normal had few diabetes related health issues after 6.5 years when compared to their individuals in conventional treatment [119,120]. Further the rate of progression of microalbuminuria to macroalbuminuria is also reduced. Similar finding was observed in the report of Microalbuminuria Collaborative Study Group [121].
The Kumamoto study, showed that the rate of conversion of microalbuminuria to macroalbuminuria was reduced with intensive treatment [113]. Perhaps the effect of strict glycemic control on progression of diabetic nephropathy are not established firmly. The oral anti-hyperglycemic agents (OHAs) are extremely helpful. Rosiglitazone, shown to reduce UAE in T2DM patients, which showed the beneficial effect in renoprotection in T2DM patients [122]. Further OHAs in proteinuric T2DM individuals, the renal function should be considered. The use of Metformin should be restricted when serum creatinine level is 1.5 mg/dl in men and 1.4 mg/dl in women, which is due to the risk of lactic acidosis [123]. Likewise, Sulfonylureas and their metabolites (except glimepiride), are excreted through renal excretion, thus it should not be used in patients with declining renal function [124]. Though, at this point, sulfonylureas and insulin promotes secretion are generally not very effective, this is due to decreased production of insulin resulting from the long duration of diabetes. Thus, most T2DM individuals with diabetic nephropathy should be treated with insulin.
Intensive blood pressure treatment and renin-angiotensin system blockade:
In T1DM and T2DM individuals with microalbuminuric, there are various studies which highlighted the treatment of hypertension, regardless of the agents used, and showed a beneficial effect on albuminuria [125]. Renin-angiotensin system (RAS) blockade along with ARBs or ACE inhibitors converses additional benefit on renal function. The blood pressure reduction is independent of Reno protective effect, which might be related to decreased intraglomerular pressure and passage of proteins into the proximal tubule [126]. Thus, these drugs which are used to reduces UAE and decreases the rate of progression of albuminuria to advance stage of diabetic nephropathy.
The ACE inhibitors reduces the risk of progression of microalbuminuria by 60% and further increases the chance of regression of microalbuminuria to normoalbumibnuria [127] among non-hypertensive microalbuminuric T1DM individuals in a meta-analysis. ARBs reduce the risk of developing macroalbuminuria among microalbuminuric T2DM individuals. In T2DM patients, the risk of progression of microalbuminuria to overt nephropathy was reduced by 70 % using Irbesartan in a 2- years follow up study. Further reduction in UAE (38%) and reversal of microalbuminuria (34%) was also observed [128]. Valsartan (80 mg/day), showed a greater reduction in UAE than amlodipine (44 vs. 8%) with the same effect of hypertension reduction [129]. Thus, the ARBs and ACE inhibitors are recommended as first line treatment for both T1DM and T2DM individuals with microalbuminuria (even in normotensive patients [130]).
The intensive treatment of HTN showed a beneficial effect in declining GFR among proteinuric T1DM individuals, which is predicted by decreasing in albuminuria [131,132]. According to the Modification of Diet in Renal Disease (MDRD) trial, decreasing blood pressure, increases the renoprotection among non-diabetic individuals [133]. The reduction in blood pressure of 125/75 mmHg, slower the rate of renal declining in patients with proteinuria (1 g/day) and renal insufficiency. Addition of ACE inhibitors and or ARBs in macroalbuminuric T1DM or T2DM individuals reduces proteinuria level and declining renal function. Though there is no significant difference in rate of cardiovascular events, it significantly lowers the rate of congestive heart failure among patients who receive ARBs [44,45].
The renin-angiotensin system (RAS) is a major regulatory system that has both cardiovascular and renal functions. The ACCORD-BP (Action to Control Cardiovascular risk in Diabetes-BP) trial showed that T2DM patients are at high risk for cardiovascular events, the target Systolic BP was
The concept of dual blockade of RAS was established by Mogensen [136], where ARBs and ACE inhibitors interrupt the RAS at various levels, and the combining these classes may add up the renoprotection effect. Other studies also showed the combined effect of ACE inhibitors and ARBs showed a synergistic effect on blood pressure and decreased UAE in patients with T1DM and T2DM individuals with diabetic nephropathy. The dual blockade of RAS showed higher impact in reducing UAE when compared to the maximal dose of ACE inhibitors [137], perhaps there is a paucity of data on the RAS dual blockade in diabetic nephropathy. In a 3 year follow up trail, (Combination Treatment of Angiotensin-II Receptor Blocker and Angiotensin-Converting-Enzyme Inhibitor in Nondiabetic Renal Disease (COOPERATE)) the dual therapy showed more effective than monotherapy in reducing the progression of renal disease [138].
Strategies of blood pressure treatment in patients with diabetic nephropathy:
In general, to achieve the target blood pressure of 130/80 mmHg in patients with diabetes / 125/75 mmHg for proteinuria (1.0 g/24 h) patients with high serum creatinine level, 3 to 4 anti-hypertensives medicated are generally used [139]. ACE inhibitors and ARBs are used as primary medication in the treatment regimen due to its well-known Reno protective effect. Individuals with 20 mmHg of Systolic blood pressure and 10 mmHg of diastolic pressure above the target goal should start with two agents. In the initial stage ACE / ARB inhibitors are used along with the low dose of thiazide diuretics, perhaps in individuals with GFR 30 ml/min and a serum creatinine level of 2.5-3.0 mg/dl, the loop diuretics should be used [116]. Both ARBs and ACE inhibitors are used in combination in patients where albumin is not reduced and or doesn’t attain the target blood pressure. Additional agents can be used as and when needed. In individuals with ACE inhibitors intolerance, ARBs are the excellent alternative and preferred agent for T2DM individuals with microalbuminuria or macroalbuminuria and or individuals with left ventricular hypertrophy [118,44]. Calcium channel blockers also shows an additional effect in decreasing blood pressure, perhaps it can be used along with the ACE inhibitors and cannot be used in patients with recent coronary events. The β-Blockers are used in individuals with myocardial ischemia, as these drugs decrease cardiovascular events and mortality rate in patients with baseline pulse rate 84 bpm [139]. Attention has to be taken when using the combination of β-Blockers and calcium channel blockers, as both have a negative chronotropic effect. The treatment should be monitored using 24 h ambulatory Blood pressure monitoring in the patients with treatment resistant hypertension, doubt of white coat hypertension, drug induced or autonomic neuropathy related hypertensive episodes [140].
Diet intervention:
Substituting chicken for red meat is the general diet which reduces UAE by 46% and decreases apolipoproteins, Total and LDL cholesterol among patients with T2DM in a 4-week time period [141]. This might be related with the reduced amount of saturated fat and increased proportion of polyunsaturated fatty acids found in chicken meat than in red meat. This reduced UAE is due to polyunsaturated fatty acids effect on endothelial function. A meta-analysis showed that the dietary protein restriction reduces the progression of diabetic nephropathy in T1DM patients in a meta- analysis [142]. A 4-year randomized control trial highlighted that the moderately low–protein diet (0.9 g/ kg/ day) reduced the risk of ESRD or mortality by 76%, although no effect on GFR decline was observed [143]. The underlying mechanism is still unclear, perhaps related to improved lipid profile and/or glomerular hemodynamics.
Dyslipidemia:
In general, the target LDL cholesterol was 100 mg/dl for individuals with diabetes, 70 mg/dl for DM for individuals with cardiovascular disease. Maintaining dyslipidemia in diabetic nephropathy is significantly important as these individuals are at high risk of cardiovascular associated mortality. As it is the major therapeutic target in diabetic treatment. The Collaborative Atorvastatin Diabetes (CARDS) Study highlighted that the cardiovascular events were reduced in individuals with diabetes with additional risk factor for CAD (coronary artery disease), thus statin it is recommended to all diabetic patients. Heart protection study highlighted the use of 40 mg simvastatin decreases the rate of vascular events and declining eGFR in patients with diabetes [144]. The treatment with Rho-kinase inhibitors along with statins shows more potent and protective on diabetic nephropathy [145]. Perhaps the contraindications of statin and fibrate use for patients with moderate to severe renal impairment should also be noted.
Anemia:
Anemia is considered as a major risk factor for progression of diabetic nephropathy and retinopathy. Erythropoietin treatment is recommended (Hb
Multifactorial intervention:
Individuals with microalbuminuria often come across with other comorbid conditions such as cardiovascular risk factors which include dyslipidemia and hypertension. Multifactorial intervention was compared with the conventional treatment in Steno-2 study, among 160 T2DM patients with microalbuminuria [150], to attain the target blood pressure (130/80 mm Hg), A1c (6.5%), fasting serum glucose (175 mg/dl) and Triglyceride (150 mg/dl) levels. Multifactorial intervention includes the gradual implementation of changes in the lifestyle of the patient, pharmacological therapy, along with the low- fat diet, light to moderate exercise (3 - 5 times / week), cessation of smoking, and administration of ACE inhibitors or ARBs and aspirin. The finding highlighted that there is risk reduction of developing macroalbuminuria (61%), retinopathy (58%) and autonomic neuropathy (63%). Further was observed that there is a significant reduction in the risk of development of composite end points including amputation, cardiovascular mortality, revascularization procedures, nonfatal myocardial infarction and stroke.
NEW POTENTIAL THERAPEUTIC STRATEGIES
The treatment plans which were discussed above might not be effective in few individuals with diabetes, thus novel therapeutic strategies are necessary. Increased dose of thiamine and its derivatives (benfotiamine) shown to reduce the development of microalbuminuria, this might be due to reduced activation of protein glycation and protein kinase C along with oxidative stress [151]. ALT-711 is a cross link breaker of AGE (advanced glycation end product), treatment with ALT-711 showed a significant reduction in blood pressure, renal lesions and UAE, in experimental diabetes [152]. The GFR was normalized using protein kinase C inhibitor (ruboxistaurin) treatment, further it reduces the albumin excretion rate and ameliorated glomerular lesions in diabetic rodents [153]. In diabetes-induced glomerulosclerosis, TFG -1 mRNA (transforming growth factor 1 mRNA) overexpression, albuminuria, glomerular and tubular matrix accumulation was prevented using modified heparin glycosaminoglycan, in rodent model [154]. There is a paucity of data in humans. A significant reduction in albuminuria was observed in micro and macro albuminuria among T1DM and T2DM patients using Sulodexide (a glycosaminoglycan) [155]. A second-generation inhibitor (AGE product) (Pimagedine) decreases the protein excretion rate and declining eGFR in T1DM proteinuric patients in randomized, placebo-controlled study [156].
CONCLUSION
Monitoring kidney function among patients with diabetic nephropathy is important to prevent further progression of the disease and also reduces the risk of heart disease. Diabetic nephropathy progression is a one-way street, thus to prevent diabetic nephropathy regularly monitoring and maintaining glycemic levels and blood pressure. Blood pressure control slows kidney and cardiovascular disease. The risk of diabetic nephropathy increases in the presence of the following conditions such as high cholesterol, high blood pressure or anemia, obese or smoking. The existing treatment of DKD relies on nephroprotective, antiproteinuric and antihypertensive effects of RAASi along with Glycemic and blood pressure control. However, this strategy is not sufficient to prevent progression to ESRD in a substantial proportion of patients. Novel biomarker panels will help to identify the patients at highest risk and guide in treatment regimens.
- Centers for Disease Control and Prevention (2020) National Diabetes Fact Sheet. Available online at: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
- Ahmad J (2015) Management of diabetic nephropathy: Recent progress and future perspective. Diabetes Metab Syndr 9(4): 343-358.
- KDOQI (2007) KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis 49(2 Suppl 2): S12-S154.
- Joslin's Diabetes Deskbook (2014) A Guide for Primary Care Providers. Boston, MA: Joslin Diabetes Center.
- Yang J, Zhang J (2015) Influence of protein kinase C (PKC) on the prognosis of diabetic nephropathy patients. Int J Clin Exp Pathol 8(11): 14925.
- Budhiraja S, Singh J (2008) Protein kinase C beta inhibitors: A new therapeutic target for diabetic nephropathy and vascular complications. Fundam Clin Pharmacol 22(3): 231-240.
- Navaneethan SD, Zoungas S, Caramori ML, Chan JC, Heerspink HJ, et al. (2021) Diabetes management in chronic kidney disease: Synopsis of the 2020 KDIGO clinical practice guideline. Ann Intern Med 174(3): 385-394.
- National Kidney Foundation (2012) KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am J Kidney Dis 60(5): 850-886.
- American Diabetes Association (2017) Standards of Medical Care in Diabetes-2017 Abridged for Primary Care Providers. Clin Diabetes 35(1): 5-26.
- Fioretto P, Stehouwer CD, Mauer M, Chiesura-Corona M, Brocco E, et al. (1998) Heterogeneous nature of microalbuminuria in NIDDM: Studies of endothelial function and renal structure. Diabetologia 41: 233-236.
- Ruggenenti P, Fassi A, Ilieva AP, Bruno S, Iliev IP, et al. (2004) Preventing microalbuminuria in type 2 diabetes. N Engl J Med 351: 1941-1951.
- Haller H, Ito S, Izzo JL, Jr, Januszewicz A, Katayama S, et al. (2011) Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med 364: 907-917.
- Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, et al. (2001) The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 345: 870-878.
- Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, et al. (2001) Reno protective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851-860.
- Macisaac RJ, Jerums G (2011) Diabetic kidney disease with and without albuminuria. Curr Opin Nephrol Hypertens 20: 246-257.
- Vijay V, Snehalatha C, Shina K, Ramachandran A (2000) Evaluation of a simple, random urine test for prospective analysis of proteinuria in type 2 diabetes: A six years follow - up study. Diabetes Res Clin Pract 49(2-3): 143-147.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Spine Diseases
- Stem Cell Research and Therapeutics (ISSN:2474-4646)
- Ophthalmology Clinics and Research (ISSN:2638-115X)
- International Journal of Anaesthesia and Research (ISSN:2641-399X)
- Journal of Cardiology and Diagnostics Research (ISSN:2639-4634)
- Oncology Clinics and Research (ISSN: 2643-055X)
- Journal of Cell Signaling & Damage-Associated Molecular Patterns


