Research Article
Pseudo Gene Signature in the Crosstalk Between Alzheimer Disease and Glioblastoma
4680
Views & Citations3680
Likes & Shares
Neurologic disorder is a prodigious phobia among people especially in elder. Alzheimer disease (AD) is a progressive neurologic disorder in brain of almost all animals and causes a generation of fearful death. On the other side, glioblastoma (GBM), a form of brain cancer, breeds in the brain cells through spreading from other parts of organ affected with cancer. A recent study predicted a conflicting outcome that informs patients with AD have a less possibility of progression of brain cancer. In continuation of this study, the present study reveals a group of collective genes that are over expressed in the brain of AD and brain cancer patients; however, overall survival analysis (OS) determines these signatory genes are not associated with the survival of GBM patients.
Keywords: Neurologic disorder, Alzheimer disease, Glioblastoma, Brain cancer, Overall survival
INTRODUCTION
AD is a progressive neurodegenerative disease characterized clinically by poor onset of memory and cognition impairment, emergence of psychiatric symptoms and behavioral disorders, and impairment of daily living activities [1]. The hallmark of pathological diagnosis of AD is characterized by the accumulations of senile plaques that are made up of amyloid-β (Aβ) peptides produced from the β-amyloid precursor protein (APP) through sequential processing by β- and γ-secretases [2,3]. The second major pathological tell-tale feature of AD is neurofibrillary tangles (NFTs) caused by intracellular accumulations of the hyper-phosphorylated microtubule-associated protein tau (MAPT) [4,5]. Neurodegeneration in AD progresses in sequential manner, generating from the predisposed induction sites in the medial temporal lobe and then gradually spreading to the entire hippocampus (Hp) and the limbic system, before advancing in topographically predictable sequences and ultimately expanding to the temporal association cortex [6]. Therefore, it might be interesting to identify possible biomarkers that cause aging-related AD in the temporal lobe.
Brain cancer is the most aggressive malignant brain tumor and more common in adults. Current treatment options after diagnosis are multimodal and include surgical resection, radiation, and chemotherapy. Unlike, other brain tumors, GBM cells have a noteworthy feature of penetrating to the surrounding brain tissue, especially in the form of a dense synaptic network and may cause synaptic and neuronal degeneration and consequently, triggered neuronal degeneration leading to neurologic deficits in vision, memory, communication, or motor functions [7-9]. The typical and exceptional responders that are involved in the development of brain cancer are currently worthy in research mind and investigating all over the world. A current study discovered a group of genes and signaling molecules that are either typically or exceptionally associated in glioblastoma [10].
In my previous recent study, it has been implicated that AD patients have a less chance of generating brain tumor [11]. To this end, I was seeking to identify common biomarkers in AD and brain tumor. The present study reveals a group of signature genes in AD and GBM patient’s brain regions; however, those genes were found not to be associated with the GBM patient’s survival.
RESULTS AND DISCUSSION
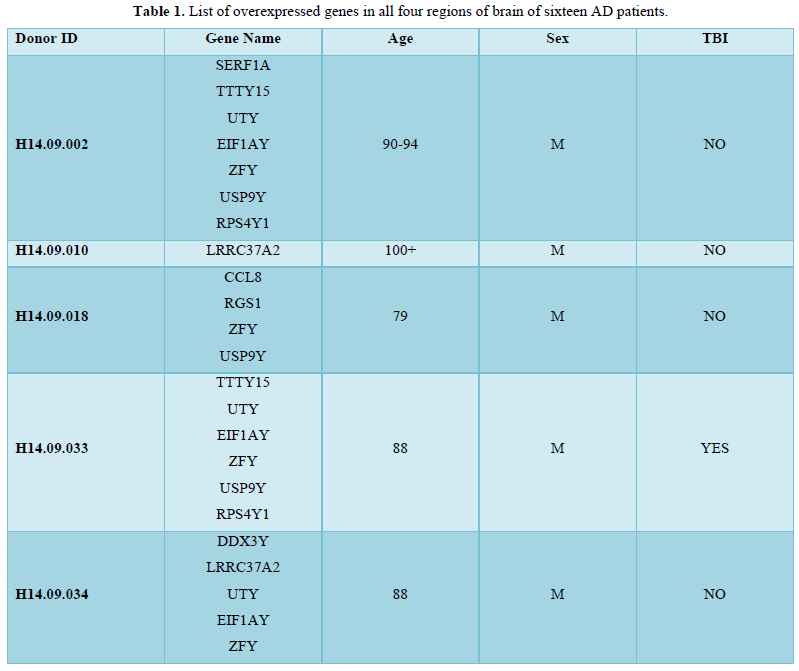
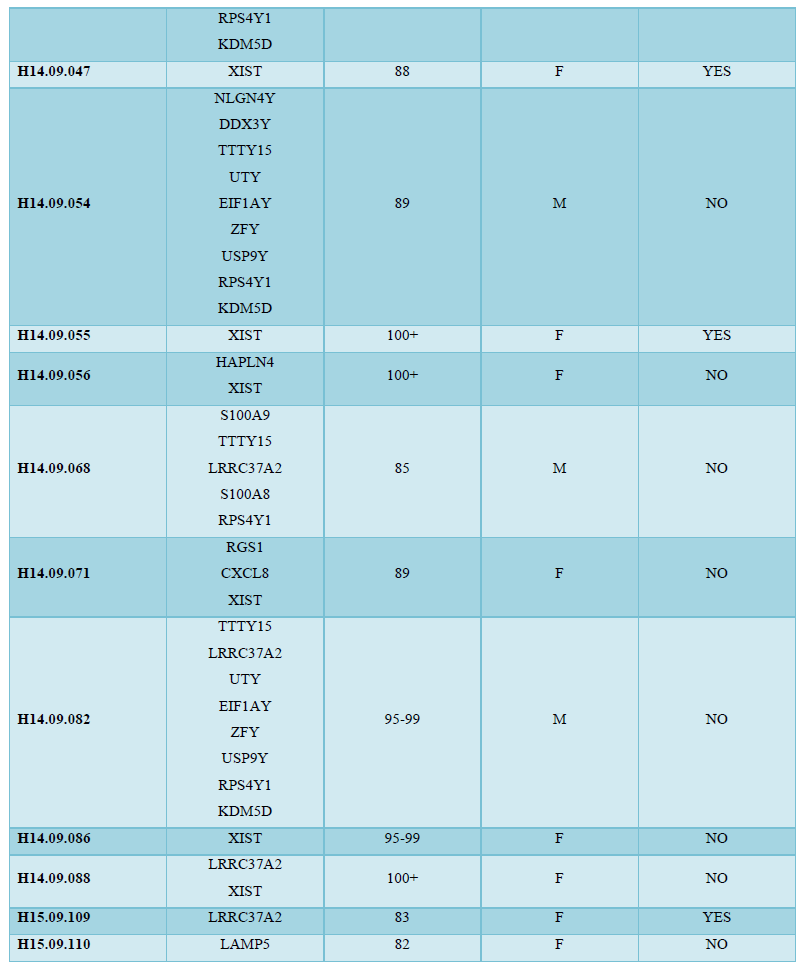
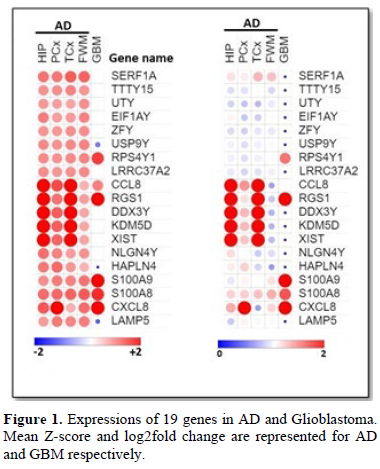
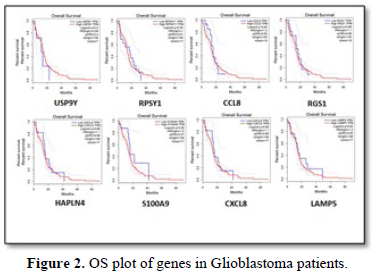
The aim of the present study was to find a group of signature genes that are responsible for the advancement of AD and GBM. To this end, a database dependent insilico study was performed. Data analysis with the database Allen Brain Atlas, thirty patients with AD were considered for the study among which sixteen patients were found to be related with overexpression of 19 genes (z score≥0.6) in all of the four regions (Hip, PCx, TCx and FWM) of brain (Table 1). The analysis was performed with all four regions of brain because of the maximum possibility of involvement of the genes that are responsible for the development of AD. Eight male and eight females from sixteen AD patients were observed to over express the 19 genes suggested that the chance factor of AD progression is not gender specific (Table 1). Moreover, patients with age ≥75 were also noticed to be diagnosed with AD (Table 1). Among these 19 genes; the expressions of 9 genes (SERF1A, TTTY15, UTY, EIF1AY, ZFY, LRRC37A2, DDX3Y, KDM5D, XIST and NLGN4Y) were found not to be significantly changed in the expressions, however, 6 genes (RPS4Y1, CCL8, RGS1, S100A9, S100A8 and CXCL8) and 3 genes (USP9Y, HAPLN4 and LAMP1) were found to be over expressed and under expressed respectively (Figure 1) in the tumor tissue of GBM patients in TCGA database in comparison with the normal tissue. Overall survival (OS) analysis was also performed with the 19 genes and almost all genes were found to not be associated with the survival of GBM patients (Figure 2). These results identified genes that are responsible for the generation of AD might not have any effect on the GBM patient’s survival although the genes could be either over expressed or under expressed significantly in the GBM tissue.
CONCLUSION
19 genes were observed to be over expressed in AD patients, among which 9 signature genes were found in GBM patients either over expressed or under expressed significantly. However, the signature genes are not associated with the survival of GBM patients. Moreover, AD is discovered as gender non-specific.
- Murphy MP, LeVine H 3rd (2010) Alzheimer's disease and the amyloid-beta peptide. J Alzheimers Dis 19: 311-323.
- Prasansuklab A, Tencomnao T (2013) Amyloidosis in Alzheimer's Disease: The Toxicity of Amyloid Beta (A β), Mechanisms of Its Accumulation and Implications of Medicinal Plants for Therapy. Evid Based Complement Alternat Med 2013: 413808.
- LaFerla FM, Green KN, Oddo S (2007) Intracellular amyloid-β in Alzheimer's disease. Nat Rev Neurosci 8: 499-509.
- Medeiros R, Baglietto-Vargas D, LaFerla FM (2011) The role of tau in Alzheimer's disease and related disorders. CNS Neurosci Ther 17: 514-524.
- Ballatore C, Lee VM-Y, Trojanowski JQ (2007) Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat Rev Neurosci 8: 663-672.
- Manavalan A, Mishra M, Feng L, Sze SK, Akatsu H, et al. (2013) Brain site-specific proteome changes in aging-related dementia. Exp Mol Med 45: e39.
- Tan AC, Ashley DM, López GY, Malinzak M, Friedman HS, et al. (2020) Management of glioblastoma: State of the art and future directions. CA Cancer J Clin 70: 299-312.
- Lim M, Xia Y, Bettegowda C, Weller M (2018) Current state of immunotherapy for glioblastoma. Nat Rev Clin Oncol 15: 422-442.
- Lukas RV, Wainwright DA, Ladomersky E, Sachdev S, Sonabend AM, et al. (2019) Newly Diagnosed Glioblastoma: A Review on Clinical Management. Oncology (Williston Park) 33: 91-100.
- Wipfler K, Cornish AS, Guda C (2018) Comparative molecular characterization of typical and exceptional responders in glioblastoma. Oncotarget 9: 28421-28433.
- Chowdhury A (2016) A Diverse Role of MMP-2 and MMP-9 in the Onset of Alzheimer Disease and Cancer. Austin Neurol Neurosci 1: 1013.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Chemotherapy Research Journal (ISSN:2642-0236)
- Journal of Ageing and Restorative Medicine (ISSN:2637-7403)
- Journal of Allergy Research (ISSN:2642-326X)
- Journal of Rheumatology Research (ISSN:2641-6999)
- International Journal of Internal Medicine and Geriatrics (ISSN: 2689-7687)
- International Journal of Diabetes (ISSN: 2644-3031)
- Advance Research on Alzheimers and Parkinsons Disease




