Research Article
Water Use, Wastewater Characteristics, Best Management Practices and Reclaimed Water Criteria in the Carwash Industry: A Review
10287
Views & Citations9287
Likes & Shares
Carwash stations use large volumes of water and release harmful chemicals into the environment through their polluted wastewaters. The type and quantity of cleaning solutions and finish products used, and the soil particles present on the vehicle have a major effect upon the characteristics of the carwash wastewater. On the other hand, global water resource supplies are worsening and as a result water shortage will affect 2.7 billion people by 2025. Therefore, understanding how much water is used by the carwash industry and the pollution loads of wastewater produced is necessary to ensure adoption of water conservation measures and to design proper wastewater treatment and recycling systems. This study showed that the amount of water used to wash a car depends on the type of washing process, type of chemical used and type of washing technology applied. There are several types of professional car washing including self-serve car washing, in-bay automatic car washing, conveyor car washing, touch-free (touchless) car washing and hybrid car washing. The characteristics of the carwash wastewater include pH, temperature, turbidity, electric conductivity, COD, BOD, solids, nutrients (ammonium, nitrite, nitrate, phosphate, and sulfate) heavy metals, oil and grease, hydrocarbons, and microbes, all of which have negative impact on human health and aquatic life. The growing public concern for water conservation, health and safety of the public water supplies and the environmental health of streams, rivers and other waterways has led to several environmental regulatory structures designed to protect drinking water and watersheds. Also, a variety of prevention measures are now available to address vehicle washing. These measures must consider the nature of the potential source of contamination, purpose, cost of operation, maintenance requirements, vulnerability of the source waters, public acceptance of the measures, and the community’s desired degree of risk reduction. There are several treatment approaches for managing and recycling wastewater, depending on the size of the site and the resources available. However, the criteria for vehicle wash reclamation systems must include public acceptance, aesthetic quality, microbiological risk and chemical issues.
Keywords: Carwash, Water Use, Water Conservation, Wastewater Characteristics, Wastewater Management, Reclaimed Water Criteria
INTRODUCTION
Car washing operations use large volumes of water and release harmful chemicals (cleaning solutions and finish products used to clean mobile vehicles) into the environment through the discharge of untreated wastewaters. Carwash wastewaters contain oil and grease (which contain hazardous materials such as benzene, lead, zinc, chromium, arsenic, pesticides, herbicides, nitrates, and other metals), detergents (that can be poisonous to fish), phosphates (which can cause excessive growth of nuisance plants in water bodies) and chemicals (such as hydrofluoric acid, ammonium bifluoride products and solvent-based solutions that are harmful to living organisms). In addition, the type and quantity of soil present on vehicles have a major effect upon the characteristics of the carwash effluent [1,2].
Greater than 99 percent of professional car washing operations in USA discharge effluent to sanitary sewers (SS) and publicly owned treatment works (POTW) [3]. Similar practices are also observed in Canada [4], United Kingdom [5], Australia [6], Brazil [7] and elsewhere [8-9]. However, only the POTW provides pretreatment guidance for discharge limits which is usually accomplished through local municipal regulations [1,3].
While a significant body of literature has focused on exploring carwash water use in the carwash industry, very little information is available on comprehensive water consumption and the pollution loads of the wastewater emanating from the carwash industry. Understanding how much water is used in the carwash industry and the pollution load of wastewater produced is necessary to ensure adoption of water conservation measures and to design wastewater recycling systems. According to Morel and Diener [10], global water resource supplies are worsening, and water shortages will affect 2.7 billion people by 2025. This means 1 out of every 3 people in the world will be affected by the water shortage problem. Thus, recycling carwash wastewater is become of paramount importance to solve this water shortages problem.
However, for carwash operators to use water wisely, they must understand what type of water being used during the carwash cycle as well as the chemical delivery and application systems. In car washing, there are three types of water used: fresh, filtered and reclaimed. Fresh water is supplied to carwash stations either through municipal means or drawn from a well. When taken from a well, the water quality can vary based on seasonal changes, pollution level in the well or water delivery system, increased or decreased levels of disinfection elements (chlorine) throughout the year and the distance between the location and the municipality water treatment plant [9]. Several technologies are used to produce filtered water and/or to treat and reclaim water.
The growing public concern for water conservation, health and safety of the public water supply and environmental health of streams, rivers and other waterways has led to several environmental regulatory structures designed to protect drinking water and watersheds [3,9]. The main aim of this study was to identify the various washing technologies used in in the carwash industry, investigate the water use to wash various types of vehicles, determine the characteristics of the carwash wastewater, and review the best management practices for car washing and reclaimed water criteria.
CAR WASHING TECHNOLOGY
The amount of water used to wash a car depends on the type of washing process, type of chemical used and washing technology. Professional car washing is generally divided into different types based on the washing technologies used. These include (a) self-serve car washing, (b) in-bay automatic car washing, (c) conveyor car washing, (d) touch-free (touch-less) car washing and (e) hybrid car washing [11].
In self-serve car washings, the car is washed by the customer. A wand disperses water and low-pressure brushes are used to clean the car. Self-serve car washing is usually coin operated system. In-bay automatic car washing, which is mostly placed at gas stations, the driver parks the car in the pay, and the coin-operated car wash-machine moves back and forth over the vehicle to clean it. In some stations, the customer pays at the pump and insert the code before the car enters the bay for washing. Conveyor car washing means full-service washing in which the exterior and interior of the car are cleaned while the customer waits outside. In this case, the car moves on a conveyor belt and friction brushes or curtains are used with soap solution and water to clean the car [12].
In touch-free (touch-less) car washing, the driver parks the vehicle in the bay and car washing high-pressure nozzles with low flow are used with soap solution and water to clean the car. While the vehicle remains stationary, one (or two) spray arm moves back and forth to clean the car. Good cleaning results depend on the effectiveness and consistent chemical application. This is the most modern vehicle wash technology in which nothing touches the vehicle except mild soap solutions and water and as a result less damage is caused to the car by the washing equipment. Moreover, the ability to measure the length and width of each vehicle reduces the consumption of water, chemical solutions, and time. A touchless cleaning in-bay requires more attention from carwash operators because the process needs suitable chemicals and high-quality soft water with optimum temperature and pressure to work smoothly [13].
Hybrid car washing combines the high pressure washing and brush washing in one operation. It is a process in which the brush washing is supported with primer prepping to reach high washing quality. Prepping is the chemical prewash (foam application) and the high-pressure water jet application is needed to rinse off contamination. Prewash before brush washing limits scratches on the car finish by effectively reducing both chemical and physical bondage between soil particles and the vehicle surface, thus, promoting removal of difficult dirt [12,13].
Williams [12] and Janik and Kupiec [13] stated that the application of modern car wash technologies results in higher cost of equipment, energy, and maintenance. Touchless and hybrid technologies are twice as expensive as traditional washing techniques because they lead to higher energy consumption due to the consumption of high-water volume (200-300 L/car) at high temperature (50°C) and high-water pressure 480-690 kPa (70-100 psi). Carwash operators by attempting to reduce operating expenses (electricity costs), they are not only benefiting themselves but also their customers and the environment.
Effective car washing technology results in high quality wash and high-water consumption. Therefore, carwash operators must balance the factors affecting cleaning including time, temperature, chemical concentration, friction of brushes or spray arm movement, energy consumption and water pressure. Some carwash investors have been able to build environmentally friendly car washing systems that provide efficient washing techniques that also allow conservation of water and energy [11,13].
Riddle [14] stated that one of the new trends to save energy in car washing is solar power. Typically, it includes solar panels on the roof of the carwash building to heat the water used for vehicle washing. Solar heating technology helps heat the water and significantly reduces natural gas consumption and CO2 emissions. Russo [15] reported that geothermal heating may also be employed as an alternative heat source that is taken from the ground around the car washing facility [15]. Wind turbine may be installed if the location of the car washing station is suitable and there is no public opposition [16].
Another method of energy reduction is using a variable-speed-drive two-vacuum pump system which is designed to nearly shut down when not in use [17]. Additionally, heat pumps and heat exchangers may improve energy savings [15]. Carwash operators can also cut energy use by installing special energy conservation doors that have an open-and-close time operation system that helps to maintain appropriate temperature in the carwash bay and conserve energy [18]. Operators can also introduce new technology for controlling drying of vehicles [11].
Utekar [19] reported on the operation of an autocar washing system using two robotic arms which was a recent trend in automation of car washing systems. One arm was mounted on the ceiling and used to clean the front and back of the vehicle with the help of wipers. The other arm along with circular brush was mounted on the longitudinal wall and used for cleaning the wheels and the sides of the car. The water required by this system is less compared to other commercial designs.
Saber [20] discussed the operation of a drying system for a rollover carwash machine using a model to describe the detachment of water droplets on solid surfaces based on airflow-imposed shear stress. This system was composed of a pair of stationary vertical dryers on the sides which were fed by two centrifugal fans, and a moveable horizontal dryer that can adjust itself to the contour of a vehicle and was connected to two centrifugal fans mounted at each end. The system replaced a portable apparatus comprising of an upright U-shaped system which moves first in one direction to wash the car and then moves in reverse direction to dry the vehicle. The new system effectively forces the water droplets downwards away from the roof and sides of the vehicle.
Tejas [21] reported on an automatic car washing and drying system in which an electro-mechanical system was used for controlling automatic car washer. The system was mainly divided into two sections namely: mechanical assembly and electrical control. The mechanism for automatic car washer includes lifting the vehicle in parallel position, moving it in forward direction, washing the vehicle firstly with foam-water then with soap-water and again with clean-water and finally lifting the vehicle again and placed it back in parallel position. The author stressed the importance of using controlling system for car washers.
Subramanian [22] reported on automatic car washing simulation using programmable logic controller (PLC). Automatic car washing includes spraying of soap solution, cleaning with water, wiping by brushes and finishing with the forced air drying. The PLC is a digitally operating electronic apparatus which uses a programmable memory for the internal storage of instructions by implementing specific functions (logic sequencing, timing, counting, and arithmetic) to control (through digital or analog input/output modules) various types of machines or processes. The PLC sends information such as enter or exit of the car and emergency instructions to the costumer through an alarm system or digital screen.
Mhaske [23] explained the use of programmable logic controller (PLC) for processing and controlling the operation of automatic car washers. Programmed auto washing framework is completely robotized with various phases of frothing, washing, drying, and brushing. The PLC is a programmable logic controller comprises of vast machines with robotized brushes controlled by legitimate controllers and it recognizes what to do through the various phases that were created and after warded into its memory.
Hossein [24] discussed the development and manufacturing of a fully automatic carwash with intelligent control algorithm using programmable logic controller (PLC). The intelligent car washer had the ability to sense the dimensions of the car and adjust parameters such as washing brush position and time duration. This automation helped to increase speed, accuracy, productivity, and safety and reduce time and cost of washing.
Vidyasagar [25] reported on the operation of an RFID-GSM autonomous car washing system and explained the use of micro-controller to process the operation of the system. The radio frequency identification (RFID) technology and the global system for mobile communication (GSM) technology are used to transfer the vehicle from the entry point to the workstation conveyer mechanism. They also described the use of various infrared (IR) sensors. A dust particle detection sensor is used to trace the dirty surface locations on car. The sprinkler and drying mechanism are used for washing and drying the vehicle.
FRESHWATER USE IN CARWASH INDUSTRY
The main objective for every carwash operator is to produce the cleanest, driest, and shiniest cars possible in a timely manner every single time a car is washed. To reach this goal, the entire carwash operation must work in synergy at all times. However, there are several critical factors that affect the car washing process and the amount of water used. These are: Water quality, temperature, mechanical action, cleaning time and chemical used. Temperature includes ambient, vehicle and solution temperatures. Ambient temperature can be controlled in the wash bay, but vehicle’s temperature can be very hot in summer, or in warmer climates, so a cool down application may help ensure that all chemicals are applied to the surface within a safe temperature range. The solution temperature can also vary from a carwash model to another. Most friction carwashes apply the ready-to-use solution at room temperature while touchless carwashes use heated water to help the chemistry work more efficiently due to less mechanical action being available, as cold water may decrease the foaming action of the cleaning solutions. In modern carwashes, there are two types of mechanical action: friction using brushes and high-pressure water. Typically, touch-free machines apply high-pressure rinses of 6,895-8,274 KPa (1,000-1,200 psi) and chemicals and solution temperature become very important to compensate for lack of friction. Time is a very important part of the cleaning process particularly when cleaning organic matter such as bugs and tree sap. The timing of equipment is imperative as it needs to work in synergy with the detergent, sealant and drying chemical solutions. The reaction between the solution and soils and the ability of the solution to remove them is also important. Alkaline cleaners (pH over 7) are the best cleaners and can be used as stand-alone cleaners to clean bugs, dirt, clays, oils, fluids, tree sap and bird droppings but it is imperative to use the correct cleaner at the correct dilution ratio [26].
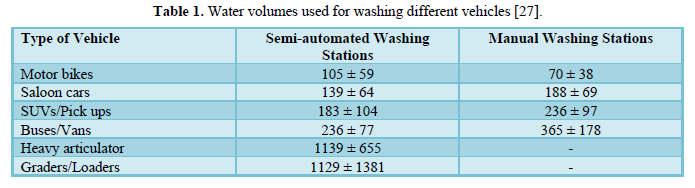
Monney [27] estimated the freshwater quantities used to wash different vehicle types and the pollution loads of the resulting wastewater in the Kumasi Metropolis, Ghana. They monitored seven carwash stations used to wash six different categories of vehicles totaling 3,667 vehicles over 8 weeks. The study showed that the amount of water used for each vehicle type was in the range of 90-103 L for motorbike, 154-161 L for salon car, 191-203 L for SUV, 351-381 L for bus, and 916-1,363 L) for truck and grader/loader. Table 1 shows the average volume of water used for washing different vehicles in semi-automated washing stations and manual washing stations.
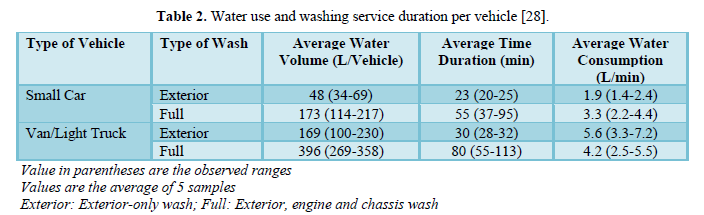
Fall [28] reported that the average water uses for exterior-only wash was 50 L per small-size car and 170 L per medium-size car (pick up, van or light truck). The average water uses for full-service wash (exterior, engine, and chassis) was 170 L per small-size car and 300 L per medium-size car. Table 2 shows water use and washing service duration per vehicle for small cars and vans/light trucks.

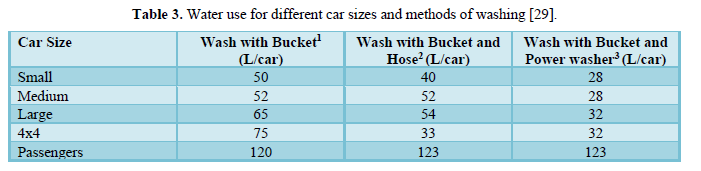
Phipps [29] stated that no attention has been paid to water use outside the house specially for car washing and conducted a study to gather information about the water used for domestic car washing activities. Table 3 shows that the use of water in individual domestic carwash is very variable and depends on the car size and method of washing. Water uses ranged from 28 L/wash to 123 L/wash. The amount of water used for passengers’ cars was higher than those used for washing other vehicles, possibly due the height of these vehicles which required extra water to wash the top. Sarne [30] compared care wash to other domestic water uses. The results shown in Table 4 indicated that toilet flushing consumes the least amount of water (15-22 L) followed by dishwasher (19-53 L), showering for 8 min (38-76 L), bath (38-152 L), professional car washing (34-157 L),

washing machine (49-190 L) and finally car washing at home (152-532 L). It is interesting to note that professional car washing consumes less water (34-157 L) than washing machine (49-190 L). Also, car washing at home (152-532 L) consumes 2-3 times more water than professional car washing (34-157 L).
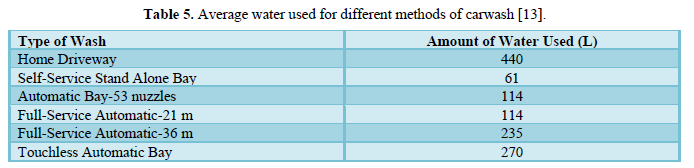
Janik and Kupiec [13] reviewed the techniques used for car washing along with issues of water use. They presented the amount of water used to wash different types of cars in Table 5 and concluded that as the water quality demand increases, viable water recycling systems become critical elements of the modern car wash facilities. Developments in technology usually lead to neutral impact on the environment. The environmentally friendly modern carwash requires a good washing technology, proper water recycling system followed by advanced water treatment methods, and compatible washing chemicals. The authors stated that developments in washing technology provide better quality of wash but lead to higher water consumption. With frequently rising water costs, they recommended recycling as much water as possible.
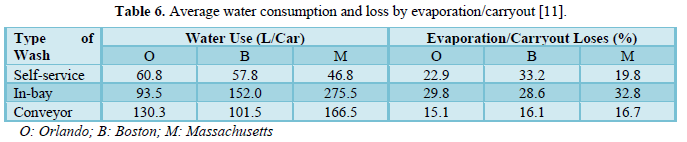
Brown [11] studied water use in the professional carwash industry. He evaluated 22 carwash sites in 3 cities (Boston in Massachusetts, Orlando in Florida, and Phoenix in Arizona). Eleven sites had some form of water reclaim systems. The reported reclaimed water as a percentage of total water used varied from 9% to 82%. The results showed that water consumption depended on the type of carwash and the region. The water consumption, water evaporation and water reclamation results are shown in Table 6. In all three regions, the in-bay carwash consumed more fresh water per vehicle. Also, Phoenix showed the highest water use per vehicle for in-bay and conveyor washing. Water losses by evaporation and carryout varied slightly among regions.

Washing was carried out by same person.
- Apply carwash from bucket with sponge and rinse off with buckets of water
- Apply carwash from bucket with sponge and rinse off with hose
- Apply carwash from bucket with sponge and rinse off with power washer.
Except for Boston with self-service washing which reported significantly higher water losses than Orlando and Massachusetts. The variation in water losses among the three types of car washing methods was very significant.
CHARACTERISTICS OF CARWASH WASTEWATER
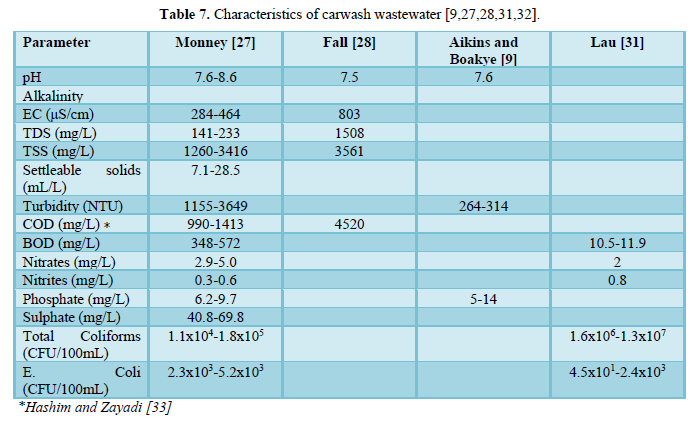
Carwash effluents contain potentially toxic chemical and microbiological pollutants (Table 7) which may pose public health and ecotoxicological threats, if directly discharged into surface waters [9,27,28,31-33]. Carwash wastewater contains solids (dissolved, suspended and settleable), oil and grease, nitrogen (ammonium, nitrite and nitrate), phosphorus (phosphate), sulfur (sulfate) as well as metals (antimony, arsenic, beryllium, cadmium, chloride, chromium, copper, lead, mercury, nickel, selenium, silver, sodium, thallium and zinc) [11,32].




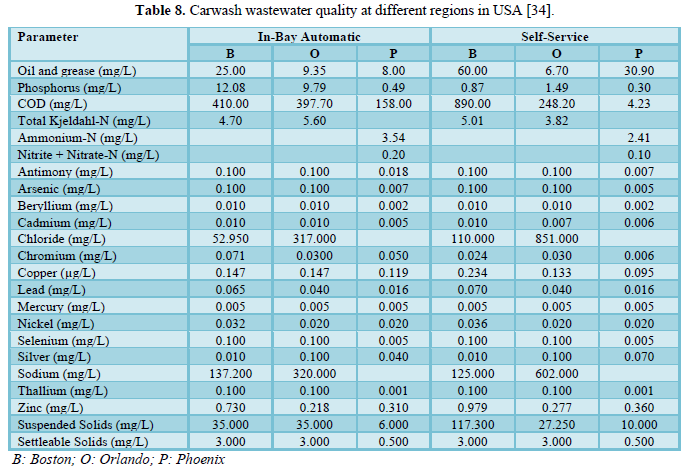
Brown [34] investigated the water quality of carwash wastewater and grit for the conveyor washers, in-bay automatic washers and self-service washing in three regions of USA (Boston, Orlando and Phoenix) which included 30 carwash operations. The results are shown in Tables 8 and 9. A comparison between the facilities in the three regions showed comparable water quality results but indicated that wastewaters from self-service car washing stations have higher levels of contaminants (oil and grease, zinc and COD) than the wastewaters from in-bay automatics and conveyor car washing stations. The grit results were reported for Boston and Orlando only and showed no significant differences based on the type of car washing facility. However, percent solids varied by regions with Boston having higher percentage (54.3%) than Orlando (35.0%) which could be due to the differences in the soil type. Other parameters that varied by location were copper, zinc, lead and oil and grease. The metals concentrations in the grit were higher than those in the local soils indicating that the cleaning detergents may be interacting with the body of the car and the tires.
Several authors reported on the various characteristics of carwash wastewaters which included: pH, temperature, electric conductivity, turbidity, COD and BOD, solids, nutrients (ammonium, nitrite, nitrate, phosphate, and sulfate), heavy metals (antimony, arsenic, beryllium, cadmium, chloride, chromium, copper, lead, mercury, nickel, selenium, silver, sodium, thallium and zinc), oil and grease, surfactants, and microbes [8,9,27,28,33,35-76].
pH
pH is a measure of how acidic or basic water is. The range goes from 0 to 14, with 7 being neutral. A pH of less than 7 indicate acidity whereas a pH of greater than 7 indicates a base. The pH of water is a very important measurement of water quality. Most aquatic creatures prefer a pH range of 6.5-9.0, though some can live in water with pH levels outside of this range. A slight change in the pH of water can increase the solubility of phosphorus and other nutrients, making them more accessible for plant growth. With more accessible nutrients, aquatic plants and algae thrive, increasing the demand for dissolved oxygen and creating a eutrophic situation (rich in nutrients and plant life but low in dissolved oxygen concentrations) in which other organisms living in the water will become stressed [35].




Monney [27] reported that carwash wastewater has a mild alkali pH in the range of 7.6-8.6. Fall [28] reported a pH in the range of 7.0-8.5 for carwash wastewater. Gorsuch [37] reported a pH in the range of 7.1-10.0 for carwash wastewater. Nguegang [38] found the pH value of the carwash effluent to lay in the alkaline range of 7-10. Sibanda [39] found the pH of all carwash effluent to be neutral to slightly alkaline. The pH values reported in the literature for carwash wastewater are within the range stated by USEPA [32] for carwash wastewater.
Hashim and Zayadi [33] obtained wastewater samples from a snow foam car wash and two full hand service car wash station and analyzed them for pH and the presence of several chemicals. The results indicated that the pH of the wastewater was slightly alkaline (7.4-8.3) and the alkalinity ranged from 21.5 mg/L to 293 mg/L.
Hickin [36] stated that the pH of water is affected by the changes occurred in it which may be due to the discharge of polluted wastewater that contains dissolved elements (Na, Cl, Ca), ions (NH4+, NO3-, NO2-, SO4--), dissolved minerals, surfactants, and dissolved gas (CO2). He also indicated that a rise in temperature and end products of biological degradation processes of organic compounds will affect the pH of water.
Aikins and Boakye [9] conducted water quality analysis on wastewater samples from carwash stations and stream water samples before discharge (upstream) and after discharge (50 m after the entry point) of the carwash wastewater in the stream over a period of 2 years in Ghana. The results indicated that the pH of the wastewater was 7.6 and there was no significant change in the stream pH as a result of carwash wastewater discharge.
Danha [40] investigated the potential impact of carwash effluent on the water quality of the receiving subtropical Nyahode river in the Eastern Highlands of Zimbabwe over one year. The pH of the effluent was in the range of 6.8-9.0 which was in the pH range of 6-9 established by the local Environmental Management Agency [41] and the World Health Organization [42] effluent standards. However, the authors stated that on site purification of wash bay effluent before discharge reduced its potential deleterious impact on water quality, river habitat integrity and aquatic biodiversity.
Rai [43] investigated the physico-chemical parameters for carwash wastewater discharged into the Olarong Chhu and Paa Chhu rivers in order to establish environmental standards for Bhutan. Their results showed that the pH of the carwash wastewater was very high (8.8-9.0). They recommended that the volume of the wastewater discharged into the surface water bodies should be monitored because if the volume and velocity of the rivers are not sufficient to handle influxes of wastewater, great environmental damage can occur. Complete analyses of physio-chemical and benthic macroinvertebrates data indicated that the ecological status of the Oarong Chhu and the Paa Chhu rivers had been degraded especially at the discharge sites where they receive untreated carwash wastewaters containing various contaminants with high concentrations.
Odeuemi [8] determined the physico-chemical parameters of water from Odo Ebo river in Nigeria with a view to provide information on the effects of discharge of carwash wastewater on the river’s water quality. The carwash wastewater had a slightly acidic pH of 5.80–7.55 which had an impact on the river water. They concluded that carwash effluents are a potential risk to the receiving water body and the government must put measures in place to check the indiscriminate siting of carwash stations by riverbanks for environmental protection.
Chukwu [44] analyzed the carwash effluents and the receiving stream water in the Niger State, Nigeria before the point of discharge and after the point of discharge. The results indicated that the wastewater effluents lowered the quality of the receiving stream water, making most of the parameters determined to be on the high side in comparison with the World Health Organization (WHO) standard [42]. The effluents were alkaline in nature and led to the precipitation of heavy metals. The authors recommended treatments of the effluents in order to make the receiving stream water safe for people living at the downstream end.
Berezin [45] investigated the influence of the pH on freshwater invertebrates and found the communities of macroinvertebrates demonstrated marked differences in their species composition and the quantitative ratios between the main groups of members (oligochaetes, chironomids, mollusks, etc.) based on the pH of the water. The highest species diversity was recorded at pH in the range of 4.09-8.65 and it decreased at a pH below 4 and above 9. The tolerance of macroinvertebrates to water pH was determined and only seven groups differing in their adaptive potential were distinguished among the 40 species found in the freshwater zoobenthos and zooperiphyton of the Upper Volga basin in Rusha.
Koryak [46] studied the bottom fauna of a stream polluted by acid mine drainage in the State of Pennsylvania, USA. The results showed that in localities immediately influenced by mine drainage, where very low pH values and high acidities prevail, the effect of acidic wastewater on the ecology and composition of the benthic fauna was similar to the effect of wastewater containing organic pollution.
Warner [47] reported that acidic water draining from coal mines has severely restricted the diversity of biota inhabiting Roaring Creek in Eastern West Virginia, USA. The polluted sections of the stream (pH ranging from 2.8 to 3.8) were inhabited by 3-12 genera of bottom-dwelling invertebrates and 10-19 species of periphytic algae. Invertebrates tolerant of the pollution included Sialis sp., Chironomus plumosus and other Chironomidae, dytiscid beetles, and Ptilostomis sp. Predominant among the tolerant periphyton were Ulothrix tenerrima, Pinnularia termitina, Eunotia exigua, and Euglena mutabilis. Six other species of algae were tolerant of the acid mine pollution but were never numerous. On the other hand, sections of Roaring Creek that were not severely polluted by acid drainage (pH higher than 4.5) supported diverse communities of more than 25 kinds of benthic organisms and more than 27 species of periphytic algae. These sections were inhibited by blackflies, crayfish, mayflies, stoneflies, and many species of caddisflies which did not inhabit the more acidic stream sections.
Temperature
Temperature plays a key role in all chemical and biochemical reactions affecting the activity of all organisms, including microbial activity needed for biological degradation processes. A temperature of 20°C is considered ideal for most biological reactions. The importance of temperature is that many of the functions and tests (BOD5, COD, DO, pH, EC and total bacterial count) are synchronized and measured with temperature. Also, water temperature affects the growth and diversity of biota inhabiting water courses [48].
When it comes to weather and temperature, a cloudy day with 20-25°C range is just about perfect for car washing. Sunny and hot or extremely cold weather can cause problems for car washing and it is not advisable to use automatic carwash at cooler temperatures. The water used for touchless and soft touch carwash should have a temperature above 10°C and the car should be warmed for 30 min. Full-service carwash stations feature heated carwash spaces and vehicle drying units. However, there is limited reporting in the literature about the temperature of carwash wastewater [11].
Odeuemi [8] reported an average temperature value of 26°C for the carwash wastewater. Rai [43] reported a temperature for the carwash wastewater in the range of 15-16°C.
Baddor [49] stated that the temperature of carwash wastewater usually is slightly higher than the temperature of a fresh water source due to the extra heat gained by water as a result of washing as well as due to the presence of pollutants. Their research aimed at investigating the treatment of a carwash wastewater taken from a licensed carwash station in the city of Aleppo, Syria by adsorption for recycling and reuse. The results showed that the effect of high temperature on treatment efficiency was insignificant and there is no need to raise the temperature to more than 20°C.
Abdel-Magid and Olabi [50] stated that temperature plays a key role in all chemical and biochemical reactions and affects the activity of all organisms including bacterial activity needed for the biological oxidation processes. They considered a temperature of 20°C to be ideal for most biological functions in nature.
El-Ashtoukhy [51] investigated the treatment of carwash wastewater produced from mobile carwash station using electrocoagulation technique and found no effect of temperature on the treatment process.
Electric conductivity
The conductivity of water refers to the ability of water to conduct an electrical current. Pure water is not a good conductor of electricity. Because the electrical current is transported by the ions in solution, the conductivity increases as the concentration of ions increases. Therefore, the electrical conductivity (EC) indicates how much dissolved substances, chemicals, and minerals are present in the water. When chemicals and salts dissolve into the water, they will turn into negatively charged and positively charged ions. The positively charged ions include potassium, magnesium, and sodium whereas the negatively charged ions include carbonate, bicarbonate, chloride, nitrate, phosphate, and sulfate. Higher amounts of these impurities lead to a higher EC [48].
Many factors affect the EC of water including water temperature, natural impacts, and human impacts. An increase in the temperature of the water by 1°C will cause an increase of EC by 2-3%. The natural factors that impact the EC of water are evaporation and rain. Some of the human impacts on water EC include agricultural runoff, road salt, septic or land fill leachate, and municipal, industrial and carwash discharges. Therefore, significant changes in the conductivity of water indicates that some pollutants have entered the water [36].
Several authors reported on the EC of carwash wastewater and its impact on natural water bodies. Nguegang [38] reported EC values ranging from 274 to 554 μS/cm for carwash wastewater. Fall [28] reported EC values for carwash wastewater in the range of 517-1070 μS/cm with an average of 803 μS/cm. Monney [27] reported EC values in the range of 284-464 μS/cm for carwash wastewater.
Danha [40] investigated the potential ramifications of carwash effluent on the water quality of the receiving Nyahode River in Zambabwe. The results showed that the control point and the off-effluent discharge source downstream points in the Nyahode river had EC values that were below the local EMA [41] and WHO [42] water quality threshold values.
Rai [43] evaluated the impact of carwash wastewater discharged into the Olarong Chhu and Paa Chhu rivers in Bhutan Kingdom and found the EC values were greater than the permissible limits of industrial wastewater in Bhutan. The data showed that the ecological status of the Oarong Chhu and the Paa Chhu rivers have been degraded especially at the discharge sites where they receive untreated carwash wastewater containing various contaminants.
Tekere [52] assessed the quality of carwash effluents from 6 carwash stations and their potential ramifications on receiving water bodies in Gauteng Province of South Africa and reported EC values in the range of 28-122 μS/cm. The authors stressed the need for the Municipal Authorities to enforce environmental by-laws governing the operations of carwash stations in South Africa which are essential in addressing the root causes of surface water pollution.
Chukwu [44] analyzed the carwash effluents and the receiving stream water before the point of discharge and after the point of discharge. The result indicated that the effluents lowered the quality of the receiving stream water, making the EC values to be on the high side in comparison with the (WHO standard [42]. The authors recommended that some treatments for wastewater be implemented to make the receiving stream water safe for people living at the downstream end.
Rai [53] conducted a survey of current washing practices and performed physico-chemical analysis of wastewaters from commercial vehicle wash centers in Olakha of Bhutan and their potential ramifications on the receiving Olarong Chhu river. The findings showed that the EC was not within the permissible limits of Environmental Standards of Bhutan and degradation in water quality at the wastewater discharging zone was observed.
Turbidity
Turbidity is the measure of relative clarity of a liquid. Material that causes water to be turbid include clay, silt, inorganic and organic matter, algae, dissolved colored organic compounds, plankton and other microscopic organisms. At no time can turbidity of water go above 5 nephelometric turbidity units (NTU). High turbidity can: (a) reduce the aesthetic quality of rivers, lakes and streams which result in harmful impact on recreation, (b) increase the cost of water treatment for drinking and food processing, and (c) harm fish and other aquatic life by degrading spawning beds, affecting gill function, killing fish or reducing their growth rate and reducing their resistance to disease [54]. Discharge of carwash wastewater into water courses can cause turbidity and as a result affect the quality of water.
However, the reported values of turbidity for carwash wastewater vary widely in the literature. Tekere [52] investigated the potential ramifications of carwash effluents on receiving water bodies and found the turbidity values for carwash effluent samples collected from six carwash outlets in Gauteng Province of South Africa to vary from 40 to 1400 NTU. Monney [27] reported high levels of turbidity in carwash wastewater and stated that the stipulated effluent discharge guideline values were mostly exceeded in some cases by up to 68 times.
Aikins and Boakye [9] conducted water quality analysis on wastewater samples from carwash stations and stream water samples before discharge (upstream) and after discharge (50m after the entry point) of the carwash wastewater in the stream over a period of 2 years in Ghana. The results indicated the turbidity of the wastewater exceeded the USEPA [25] effluent limits and there was a significant increase in the turbidity 50 m downstream as a result of carwash wastewater discharge.
Odeuemi [8] determined the physico-chemical parameters of water from Odo Ebo river with a view to provide information on the effects of discharge of carwash wastewater on the river’s water quality. They reported turbidity values for carwash wastewater in the range of 44-89 NTU and attributed the high turbidity values recorded at the discharge points to the color of detergent and oil/grease in the carwash wastewater.
Rai [53] conducted a study comprising of a survey of current washing practices and physico-chemical analysis of wastewaters from commercial carwash centers and their potential ramifications on receiving Olarong Chhu river in Olakha, Bhutan. The findings showed that vehicle wash centers were operated with poor environmental ethics and the turbidity of the effluent was estimated at about 208 NTU. Degradation in the water quality of the Olarong Chhu river, especially at wastewater discharging zone, was observed.
Al-Gheethi [55] stated that carwash wastewater has high turbidity level (275 NTU) and as such represents one of the heavily contaminated wastewaters with high impurities due to the presence of sand and particles, oil and grease, surfactants, detergent, phosphates, and hydrofluoric acid. They found that direct disposal for high turbidity carwash wastewater into the water courses represents a burden on the sewage treatment plant due to the presence of materials that caused the turbidity.
Mohammadi [56] stated that carwash wastewaters have high turbidity (170 NTU) due to the presence of numerous pollutants including heavy metals, detergents, surfactants, and organic matter and can be harmful to human and environment. They found biological treatment to have limited ability to treat many carwash wastewaters due the presence of high concentration of heavy metals.
BOD and COD
The biological oxygen demand (BOD) is an important parameter for determining the amount of biodegradable organic pollutants in water by measuring the amount of oxygen required to break down these organic substances by microbial reactions. Testing BOD has its widest application in measuring waste loadings of treatment plants and in evaluating treatment efficiency. BOD is a definitive indicator of the required treatment of wastewater and estimating BOD is an important part of wastewater treatment process control. BOD testing uses microorganisms that consume oxygen while feeding on the organic compounds in a wastewater sample over a five-day period. While this test is a good model of the aerobic waste treatment process, in some cases the microorganisms can become poisoned by toxic substances in the untreated wastewater. However, the five-day BOD test does not provide the real-time information necessary to make process control decisions [48,57].
The chemical oxygen demand (COD) is a measurement of the amount of oxygen required to break down pollutants (organic substances) in water by chemical reactions. Many wastewater treatment facilities use COD test to estimate BOD level as the COD is complete in two hours, and the reproducible COD result correlates with BOD. Toxic materials in the sample do not affect the oxidant, so the COD test provides a good indicator of organics in industrial wastewater containing heavy metals and cyanides. COD testing assesses all chemically oxidizable substances and can be directly related to the true oxygen demand imposed by the effluent if released into the environment. Because each organic compound differs in the amount of oxygen necessary for complete oxidization, the COD test reflects the effect of an effluent on the receiving stream more directly than the measurement of carbon content [57,58].
The BOD:COD ratio is an important tool in evaluating wastewater treatment. The ratio of BOD:COD indicates the biodegradability of organic materials in wastewater. A high BOD:COD ratio indicates that the organic compounds are easily oxidized. A shift in the BOD:COD ratio in the influent means a change in the type of organic compounds entering the system, which can impact the effectiveness of the process. Discharge of a wastewater high in BOD:COD ratio into water bodies will have serious health and environmental impact [48,57,58].
Several authors reported on the BOD: COD values of carwash wastewaters and the impact of the pollution load on the quality of water courses. Odeuemi [8] investigated the effect of discharge of carwash wastewater on the Odo Ebo river’s water quality. The results showed that the wastewater had high levels of COD and BOD5 at the effluent receiving points which also resulted in higher biological productivity and organic detritus.
Monney [27] estimated the pollution loads resulting from carwash wastewater by analyzing composite wastewater samples collected from three carwash stations involving 3,667 vehicles over a period of 8 weeks in the Metropolis of Ghana. Overall, the carwash industry in the Metropolis uses about 1000 m3 of freshwater daily and discharges the resulting wastewater into waterways. The carwash wastewater had a low biodegradability Index (0.3-0.4) and high levels of COD (990-1413 mg/L) and BOD (348-572 mg/L). Pollution loads of BOD and COD were up to 2 and 6 tons/year, respectively. The study recommends enforcement of wastewater treatment and recycling for all carwash stations.
Fall [28] reported that the carwash wastewater had a low biodegradability index (0.35) and high COD levels (897-7814 mg/L) with an average value of 4520 mg/L. Their results indicated that the pollution loads of BOD and COD exceeded the stipulated effluent discharge guideline values by up to 68 times in some cases. The study recommends enforcement of wastewater recycling for all carwash stations and promulgation of a tax system that rewards stations recycling wastewater and a surcharge for stations wasting freshwater.
Hashim and Zayadi [33] stated that huge quantities of water are being consumed during washing cars and the resulting untreated effluents are discharged to the stormwater system. The study reported a COD average value of 485 mg/L for wastewater samples obtained from several carwash stations.
Danha [40] investigated the potential ramifications of carwash effluent released off Charter Estates, Chimanimani in the Eastern Highlands of Zimbabwe on the water quality of the receiving subtropical Nyahode river by measuring biological oxygen demand (BOD), chemical oxygen demand (COD), dissolved oxygen (DO) over 2-year period. The results show that the control point and the off-effluent discharge source downstream points in the Nyahode river had water quality parameters that were below the local Environmental Management Agency (EMA) [41] and the World Health Organisation (WHO) [42] water quality threshold values. They concluded that Nyahode river has a functional self-purification capacity.
Chukwu [44] analyzed carwash effluents and the receiving stream water in the Niger State of Nigeria before the point of discharge and after the point of discharge. The results indicated that the effluents lowered the quality of the receiving stream water, making most of the parameters determined including COD and BOD to be on the high side of the WHO standards [42]. The dissolved oxygen was in the range of 1.41-4.30 mg/ L while the BOD was between 2.18 mg/ L for upstream and 4.70 mg /L at the point of the discharge. The COD was between 4.0 and 5.20 mg/L for downstream and point of discharge, respectively. Some treatments measures for the effluents were recommended to make the receiving stream water safe for people living at the downstream end.
Rai [53] analyzed wastewaters from commercial vehicle wash centers located at Olakha and investigated their potential ramifications on receiving Olarong Chhu river in Bhutan. The analyses of Olarong Chhu river depicts high BOD5 and COD values that resulted in the degradation of water quality especially at the wastewater discharging zone.
Solids
Total solids (TS) in wastewater are made of dissolved solids (DS), suspended solids (SS) and settleable solids, all are measured in mg/L. Sources of total solids in water bodies include industrial and carwash wastewater discharges, sewage, fertilizers, road runoff, and soil erosion. Higher levels of total solids will: (a) make drinking water unpalatable and might have an adverse effect on people health (b) affect water clarity and decrease the passage of light through water, thereby slowing photosynthesis by aquatic plants, (c) make water to heat up more rapidly and hold more heat which might adversely affect aquatic life and (d) reduce the efficiency of wastewater treatment plants and the operation of industrial processes that use raw water [48,57].
Dissolved solids consist of chlorides, fluoride, calcium, magnesium, sodium, potassium, iron, nitrate, sulfate, phosphate, carbonate, bicarbonate, organic acids, and other particles that will pass through a filter with pores of 2 microns in size. An organism placed in water with a high concentration of solids will shrink somewhat because the water in its cells will tend to move out which will in turn affect the organism's ability to maintain the proper cell density, making it difficult to keep its position in the water column and as a result it might not survive [48,57,58].
Suspended solids include silt and clay particles, plankton, algae, fine organic debris, and other particulate matter that will not pass through a 2-micron filter. Higher concentrations of suspended solids can: (a) serve as carriers of toxic substances, which readily cling to suspended particles and (b) clog irrigation devices [48,57].
Settleable solids are those solids that will settle to the bottom of an Imhoff cone in a given time period. In the laboratory analysis, the mixed water sample is quickly poured into an Imhoff cone and allowed to stand undisturbed for 60 min. The test is useful for determining the amount of solid entering a wastewater treatment plant, as well as for estimating the amount of sludge to be expected during the treatment process. Heavier particles, such as gravels and sand, often settle out when they enter an area of low water flow. Although this settling improves water clarity, the increased silt can smother benthic organisms and eggs [48,457,58].
Brown [34] stated that untreated carwash wastewater effluents contain total, dissolved, suspended, settling solids and their discharge into water bodies will affect water quality and impair its use for recreational, industrial, and municipal uses as well as impact aquatic life.
Several authors reported on the concentrations of solids in carwash wastewaters. Monney [27] estimated the pollution loads resulting from carwash wastewater and reported the values of 1260-3418 mg/L, 142-233 mg/L and 7-28 mg/L for the TSS, TDS and settleable solids, respectively. Fall [28] reported high levels of TSS and TDS for carwash wastewater of 3561 mg/L (905-4887 mg/L) and 1508 mg/L (728-2442 mg/L), respectively. Hashim and Zayadi [33] stated that huge quantities of water consumed during washing cars yields untreated effluents discharged to the stormwater system with a TSS concentration of 325mg/L.
Odeuemi [8] analysed the carwash effluent discharged to OdoEbo river and found high concentrations of TSS and TDS. The authors attributed the increase in loads of the major ions in the DS (phosphate, sulphate, and nitrate) to the hydrocarbon components of detergents used at the carwash operations.
Aikins and Boakye [9] conducted water quality analyses on wastewater samples from carwash stations and stream water samples before discharge (upstream) and after discharge (downstream 50 m after the entry point) of the carwash wastewater in the stream over a period of 2 years in Ghana. The results indicated that the TDS and TSS of the wastewater exceeded the USEPA [32] effluent limits for discharge into watercourse. Also, analyses of the samples collected downstream indicated significant increase in TDS and TSS as a result of carwash wastewater discharge.
Danha [40] investigated the potential ramifications of carwash effluent on the water quality of the receiving Nyahode River in Zimbabwe. The TSS and TDS of the river water were below the local Environmental Management Agency (EMA) [41] and World Health Organization (WHO) [42] water quality threshold values.
Rai [43] reported that the concentrations of TSS and TDS (including Cu, Fe, and Mn) in carwash wastewater discharged into the Olarong Chhu and Paa Chhu rivers in Bhutan were greater than the permissible limits of industrial wastewater. The results indicated that the ecological status of the Oarong Chhu and the Paa Chhu rivers had been degraded especially at the discharge sites.
hukwu [44] analyzed the carwash effluents and the receiving stream water before the point of discharge and after the point of discharge and found the TSS and TDS of the effluents to be on the high side (8.1-118.6 mg/ L) in comparison with the WHO standard [42] and as a result lowered the water quality of the receiving stream. The levels of heavy metals were also high. Iron was 3.21-3.27 mg/L and chromium was 0.20-0.27 mg/L which were above the recommended permissible levels for drinking water.
Rai [53] performed physico-chemical analyses of wastewaters from commercial vehicle wash centers located in Olakha and evaluated their potential ramifications on the receiving Olarong Chhu river in Bhutan. The results showed that the TSS (364.29 mg/L), TDS (204.45mg/L) and dissolved heavy metals (Cu=0.11 mg/L, Fe =15.06 mg/L and Mn=0.73 mg/L) were not within the permissible limits of Environmental Standards of Bhutan which resulted in the degradation of the water quality of the river especially at the wastewater discharging zone.
Nutrients
Carwash wastewater is rich in nutrients which include ammonium, nitrite, nitrate, phosphate, and sulfate. Although these nutrients are beneficial to plant life, high concentrations can result in adverse effects on streams, rivers, lakes, and coastal waters resulting in serious environmental and human health issues and impacting the economy [47]. High concentrations of these nutrients in the water causes algae to grow faster than ecosystems can handle. This leads to eutrophication of the receiving surface waters causing oxygen deficiency that result in fish kill, decay of aquatic life and changes in the color and taste of the water [48,53].
Several studies reported on the levels of various nutrients in carwash wastewaters and the impact of their discharge to surface water. Atkins and Boakye [9] reported phosphate concentration levels in carwash wastewater that exceeded USEPA [32] effluent limits for discharge into watercourse and indicated that the discharge of carwash wastewater in stream resulted in a significant increase in phosphate concentration of the stream water causing eutrophication.
Odeuemi [8] stated that the discharge from carwash effluent had high concentrations of essential nutrients (nitrate, nitrite, sulfate, and phosphate) which led to increases in the primary productivity (abundance of zooplankton) of the water body causing increases in turbidity and reduction in dissolved oxygen.
Monney [27] estimated the pollution loads of the resulting wastewater in the Kumasi Metropolis of Ghana. The results showed the carwash wastewater contained 40.8-69.8 mg/L sulphate, 6.2-9.7 mg/L phosphate, 0.3-0.6 mg/L nitrite and 2.9-5.1 mg/L nitrate.
Hashim et and Zayadi [33] analyzed wastewater samples collected from several carwash stations at different stages of washing and found the presence of PO4--- to be the highest (10.18 mg/L,) in the wash stage and NO3- and NO2- to be the highest (0.4 mg/L nitrite and 3.9 mg/L nitrate) in the rinse stage.
Danha [40] investigated the potential ramifications of carwash effluent on the water quality of the receiving Nyahode River in Zimbabwe. They found the nutrients (ammonium, nitrite, nitrate and phosphate) levels in the river were below the local Environmental Management Agency (EMA) [41] and World Health Organization (WHO) [42] water quality threshold values.
Tekere [52] investigated the physicochemical quality and potential ramifications of carwash effluents on receiving water bodies in Gauteng Province of South Africa. The analyses of effluent samples collected from 6 carwash outlets showed ammonium concentrations ranging from 0.4 to 75 mg/L which were above the local Environmental Management Agency stipulated guidelines [41].
Rai [53] performed physico-chemical analyses of wastewaters from commercial vehicle wash centers located at Olakha, Bhutan and investigated their potential ramifications on receiving the Olarong Chhu river. The results showed that total nitrogen and total phosphorous were not within the permissible limits of Environmental Standards of Bhutan and degradation in water quality was observed especially at wastewater discharging zone due to the increased loads of the major ions (nitrite, nitrate, phosphate and sulphate) from the hydrocarbon components of detergents used at the carwash operation.
The City of Durham [59] performed a study to quantify pollutant loads associated with mobile commercial car washes. The study revealed that several pollutant concentrations in mobile commercial carwash wastewaters were similar to or higher than untreated domestic wastewater. The annual total nitrogen and phosphorus loads were 14.5 kg/year and 2.4 kg/year, respectively.
Malinen [60] reported total phosphorus and total nitrogen in carwash wastewater of 7 mg/L and 25 mg/L, respectively. They suggested that these nutrients could be utilized by microbes in a biological treatment of car washing wastewaters.
Heavy metals
Carwash wastewater have been reported to contain several heavy metals including antimony (Sb), arsenic (As), barium (Ba), beryllium (Be), cadmium (Cd), chloride (Cl), chromium (Cr), copper (Cu), iron (Fe), lead (Pb), manganese (Mn), mercury (Hg), nickel (Ni), selenium (Se), silver (Ag), sodium (Na), strontium (Sr), titanium (Ti), thallium (Tl) and zinc (Zn). As such, heavy metal toxicity can have several consequences on the human, animal, plants, and aquatic organisms [61].
Uptake of heavy metals by plants and subsequent accumulation along the food chain is a potential threat to animal and human health. For human and animals, it can affect the central nervous function leading to mental disorder, damage the blood constituents, lungs, liver, kidneys, and other vital organs thereby promoting several disease conditions. Heavy metal overload has inhibitory effects on the development of aquatic organisms (phytoplankton, zooplankton, and fish) [62,63]. The metallic compounds could disturb the oxygen level and mollusks development, byssus formation, as well as reproductive processes [62-64].
Aikins and Boakye [9] conducted water quality analysis on carwash wastewater and found Pb, Zn, Cu and Fe in higher concentrations that exceeded USEPA [32] effluent limits for discharge into watercourses. The results also showed significant increases in the concentrations of all heavy metals in the stream water as a result of wastewater discharge.
Nguegang [38] determined the physicochemical, and toxicological parameters of carwash effluents as well as the toxicity assays using Danio rerio and Daphnia pulex. Concentrations of Co, Pb, and Ni were < 1 mg/L in all samples while the concentration of Cu ranged from 0.94 to 3.8 mg/L and the concentration of Zn ranged from 1.15 to 3 mg/L. All the carwash effluents were categorized as acutely toxic, with ≥ 75% mortality recorded for both test organisms within the first 24 h of exposure to the test solutions. The authors concluded that carwash effluents are sources of potentially toxic chemical pollutants of public health and are of ecotoxicological concern.
Rai [43] reported that the concentrations of, Cu, Fe, and Mn in carwash wastewater discharged into the Olarong Chhu and Paa Chhu rivers in Bhutan were greater than the permissible limits of industrial wastewater. The data showed that the ecological status of the Oarong Chhu and the Paa Chhu rivers have been degraded especially at the discharge sites. They suggested that the volume and pollutants load of the wastewater discharged into the surface water bodies should be monitored.
Chukwu [44] analyzed the carwash effluents and the receiving stream water before and after the point of discharge. Their results showed that the levels of heavy metals were high. Iron was found to be between 3.21 and 3.27 mg/L, which is above the recommended permissible levels for drinking water. Chromium was found to be between 0.20 and 0.27 mg/L. The results indicated that the effluents lowered the quality of the receiving stream water.
Tekere [52] investigated the physicochemical quality and potential ramifications of carwash effluents on receiving waterbodies in South Africa. Effluent samples were analyzed for zinc, copper, lead and chromium, and their toxicity potential were assessed using organisms from four trophic levels ranging from Selenastrum capricornutum (primary producer), Daphnia magna (primary consumer), Poecilia reticulata (secondary-tertiary consumer) and Vibrio fischeri (decomposer). High pollutant levels for Zn (0.79 - 20.12 mg/L), Cu (0.77 13.71 mg/L) were recorded. Toxicity assessment assays resulted in 100 % mortality for fish and Daphnia after 96 and 24 h, respectively. Significant bioluminescence and growth reduction in V. fischeri and algae were observed after 15 min and 72 h, respectively. Additionally, the effluents demonstrated acute toxicity against all four test species.
Rai [53] determined the physico-chemical parameters of wastewaters from commercial vehicle wash centers located in Olakha, Bhutan and evaluated their potential ramifications on the receiving Olarong Chhu river. The results showed that Cu (0.11 mg/L), Fe (15.06 mg/L) and Mn (0.73 mg/L) were not within the permissible limits of Environmental Standards of Bhutan.
Abagale [65] assessed the concentration of heavy metal in carwash wastewater from several carwash stations in Ghana. The results indicate that concentration levels of Cr (0.42 mg/L), Fe (4.97 mg/L), Pb (0.28 mg/L) and Mn (2.36 mg/L) in the samples were high whereas the concentrations of Zn (0.18 mg/L), Cd (0.002 mg/L) and Cu (0.06 mg/L) were low. It was also observed that the concentrations and occurrence of Fe and Mn were inter- related whilst the concentration of Mn was inter-related with the concentrations of Zn, Cu, Cd, Cr and Pb. Based on these results, the authors stated that extreme caution must be taken when using carwash wastewater for crop irrigation and bioaccumulation of these heavy metals in the soil and their effect on stream water should to be checked out regularly.
Qu [66] characterized the responses of benthic macroinvertebrates to heavy metals released from mines in high mountain streams of the Gangqu river in the Shangrila Gorge, China. Benthic macroinvertebrates were collected with a kick-net at 32 sampling sites and heavy metal concentrations were measured at each sampling site. Although the concentrations of heavy metals were not seriously high, their effects were reflected in the changes of community composition of benthic macroinvertebrates. Total abundance and species richness decreased with increasing heavy metal concentrations. Species richness index, and percentage of scrapers in functional feeding groups were negatively correlated with heavy metal concentrations.
Tao [67] assessed the distribution and bioaccumulation of heavy metals in aquatic organisms of different trophic levels and the potential health risk. Heavy metals (Cu, Zn, Cr, Ni, Cd, Pb) were measured in phytoplankton, zooplankton, in two species of zoobenthos, and in eight fish species as well as in the water column and bottom sediments of Taihu lake. The results showed that the concentration of Cu and Zn for all organisms was much higher than for other metals, and Cd was the lowest in all species. Generally, heavy metal concentrations in phytoplankton were higher than in zooplankton. In zoobenthos, the concentration in Bellamya sp. (human edible snail) was higher than that in Corbiculidae (bivalve). Metal concentrations had no significant difference between fish species but tended to be higher in predator fish such as Coilia ectenes and Erythroculter ilishaeformis than in herbivorous fish.
Oil and grease
Carwash wastewater is a source of oil and grease. Oil and grease include fats, oils, waxes, and other related constituents found in carwash wastewater. If these compounds are not removed before discharge of untreated carwash wastewater, they can combine with other toxic substances and interfere with biological life in surface waters and create unsightly films. These contaminants contribute to the destruction of natural aquatic habitats and the live in those habitats by exposing aquatic organisms to harmful chemicals that would otherwise not be present over the natural water course [68].
Fall [28] reported oil and grease levels in carwash wastewater in the range of 404-2876 mg/L with an average value of 1099 mg/L. Sibanda [39] evaluated the quality of carwash effluents and found the oil and grease concentrations in the range of 15.3 - 49.7 mg/L.
Tekere [52] investigated the physicochemical quality of carwash effluents in South Africa and found high levels of oil and grease (12.8-43.1 mg/L) in the wastewater which were above the USEPA [32] stipulated guidelines.
Aikins and Boakye [9] conducted water quality analysis on wastewater samples from several carwash facilities in Ghana and found the wastewater to contain high levels of grease and oil which exceeded the USEPA [32] effluent limits for discharge into watercourses. They concluded that the discharge of the untreated carwash wastewater into streams had a negative impact on human health and environment.
Hashim and Zayadi [33] obtained wastewater samples from carwash station and analyzed them for the presence of oil and grease. The results indicated that the highest concentration of oil and grease (85.64 mg/L) was found in the pre-soak stage.
Nguegang [38] determined the physicochemical and toxicological parameters of carwash effluents and fond low concentrations (< 1 mg/L) of total petroleum hydrocarbon-gasoline range organics (TPH-GRO) in all samples.
Danha [40] determined the oil and grease content in carwash wastewater Zimbabwe. The results indicated that carwash effluent had high concentration of oil and grease and recommended an onsite purification process of carwash effluent before discharge to water bodies to reduce its potential deleterious impact on water quality and aquatic biodiversity.
Rai [53] conducted a study on carwash wastewaters from commercial vehicle wash centers located at Olakha in Bhutan and the results showed that carwash effluent oil and grease level (154.57mg/L) was not within the permissible limits of Environmental Standards of Bhutan.
Surfactants
Synthetic surfactants are components of household and industrial detergents [69]. Surfactants are organic chemicals that adsorb at the interface between air and water or at the interface between oil and water in the case where water is mixed with oil. In recent years, surfactants have widely been used in many industries and daily life for their interfacial functional capabilities. Carwash wastewaters have been reported to contain significant concentrations of surfactants [70].
Perkowski [71] stated that surfactants belonging to those compounds which are commonly used in carwash operations and have a great impact on water pollution. In recent years, a considerable increase in surfactant usage and significant enhancement of surfactant concentration in carwash wastewater have been observed, reaching even several hundreds of mg per liter. In addition, the car washing installations, which produce detergent-rich wastewaters, are usually located together with petrol stations, many of them are placed far away from urban area and are facing serious problems of wastewater disposal.
After utilization in car washing, large quantities of surfactants and their derivatives in carwash wastewater are discharged into aquatic and or terrestrial environments [72]. These compounds can cause problems in treatment facilities due to their high foaming capabilities, lower oxygenation potentials and consequently kill waterborne organisms [73]. The rapid removal of these compounds from the environment to avoid secondary pollution will make their applications safer and more widespread [74].
Hashim and Zayadi [33] obtained wastewater samples from a snow foam carwash and two full hand service carwash stations and analyzed them for the presence of surfactant in carwash wastewater. They found the highest concentration of surfactant (54.00 ± 2.50 mg/L) to be in pre-soak stage.
Shabbazi [70] stated that sodium dodecyl sulfate (SDS) is one of the main surfactant components in detergents used in high amounts in shampoos and carwash soap and proposed its bioremediation by suitable microorganisms. They isolated SDS-degrading bacteria from Tehran city carwash wastewater and used it to study bacterial alkyl sulfatase enzyme activity and identify the alkyl sulfatase enzyme coding gene. The isolated SDS-degrading bacterium showed valuable biodegrading potentials. The results showed a maximum SDS degradation of 84% by P. aeruginosa KGS in basal salt medium containing 1.5 mM SDS at pH of 7.1, temperature of 30°C and agitation at 150 rpm after four days incubation.
Hosseini [75] reported that sodium dodecyl sulfate (C12H25OSO3Na) is an essential foaming agent and component of carwash soap and shampoos. It has two units: a hydrocarbon chain (C12) and a sulfate group attached to the chain. Due to the increased use of SDS, its bioremediation by suitable microorganisms has gained much importance [75-77].
Bakacs [78] reported that many groups hold car washing fundraisers unaware of pollution issues associated with carwash runoff which can be a pollution source to surface water bodies. Their preliminary study revealed that rain gardens can be an appropriate management practice for reducing carwash surfactants by 89-96%. However, the removal efficiencies for surfactants were not enough to reduce their concentrations below the reported aquatic toxicity levels.
Linclau [79] reported that wash water streams coming from rinsing of equipment in a detergent production site is in considered as waste. They developed a new treatment approach based on nanofiltration technology in a detergent production site in China to split wash water into a concentrate stream that contains most of the valuable surfactants that can be recycled, and a water fraction that can easily be polished by membrane bioreactor and to feed the cooling towers.
Microbes
Several authors reported on the presence of various microbes in carwash wastewater. These microbes included total coliform, E. Coli, Aeromonas, Pseudomonas. Proteobacteria, Bacteroidetes, Firmicutes, Acidobacteria, and Verrucomicrobia [7,27,28,38,39].
Zaneti [7] evaluated carwash wastewater and discussed the results in terms of aesthetic quality (water clarification and odor), health (pathological) and chemical (corrosion and scaling) risks. By applying microbiological risk model, the Escherichia coli proposed criterion for carwash reclaimed water was 200 CFU/100mL. They believed that the carwash wastewater reclamation criteria may assist institutions to create laws related to carwash wastewater reclamation and discharge criteria in Brazil.
Monney [27] estimated the microbial and pollution loads of the wastewater resulting from carwash stations in the Kumasi Metropolis of Ghana. They found the wastewater to contain high levels of total coliform (1.1x104-1.5x105) and E. coli (2.3-4.7 × 103 CFU/100mL). The study recommends enforcement of wastewater treatment and recycling for all carwash stations.
Fall [28] reported the presence of E. coli in carwash wastewater at a concentration of 2.3-4.7 × 103 CFU/100mL. Their study recommended enforcement of treatment and recycling of wastewater for all carwash stations.
Nguegang [38] investigated the physicochemical and microbial parameters of carwash effluents and found heterotrophic bacteria count in range of 2800 - 4600 CFU/100mL. Sequencing analysis revealed that 57% of the isolates were closely related to Aeromonas species and 43% closely related to Pseudomonas species. They concluded that carwash effluents are veritable sources of microbiological contaminants that are of concern to public health.
Sibanda [39] investigated the bacterial diversity in carwash effluents to determine their potential for use in microbial degradation of environmental contaminants. Nine carwash effluent samples were collected for bacterial community diversity analysis using multi-digital probes and 16S rRNA gene amplicon sequencing. 16S gene amplicon sequencing of the nine samples produced 45,934-sequence reads, which translated to 13 bacterial phyla, 26 classes, and 43 genera. The most dominant phyla were Proteobacteria, Bacteroidetes, Firmicutes, and Fusobacteria. Canonical correspondence analysis (CCA) showed that the distribution of the phyla Proteobacteria, Bacteroidetes, Acidobacteria, and Verrucomicrobia was influenced by the presence of oil and grease, total petroleum hydrocarbons-gasoline range organics (GRO-TPH), and metals species (Pb, Cu, and Zn).
RECLAIMED WATER CRITERIA
Asano [80] stated that the WHO [42] and USEPA [81] guidelines for reclaimed water are far too stringent and emphasized the need for less conservative standards. However, water criteria for vehicle wash reclamation systems must include public acceptance, aesthetic quality, microbiological risk, and chemical issues.
Jefferson [82] investigated public acceptability of water reuse in urban modalities in England. The results showed that wide acceptance has been reported for vehicle wash wastewater reuse with the strict control of aesthetic (odorless, and low turbidity) and microbiological (low health risk) quality.
Brown [7,83] in his reports for the International Carwash Association indicated that the water quality for vehicle wash must be sufficiently high such that vehicles and wash equipment are not damaged (corrosion, scaling, spots and chemical risk), the risk to operators and users is minimal (microbial risk), and the aesthetic conditions are acceptable.
Hass [84] stated that the microbiological risk of carwash wastewater, which is a major concern, has not yet been measured. This risk may be estimated by quantitative microbial risk assessment (QMRA), defined as the application of the principles of risk assessment to estimate the consequences of a planned or actual exposure to infectious microorganisms. QMRA has been applied to establish standards, guidelines and other recommendations regarding drinking water and water reuse and can be performed using Dose Response Models [85].
Asano [80] and Zeneti [86] indicated that the presence of heavy metals in wastewater and the addition of chemicals in wastewater treatment, including chlorine to disinfect reclaimed water, increases the salt concentration which may build up to the point where the reclaimed water becomes unsuitable for reuse.
However, Smith and Mleziva [87] and Heidler and Halden [88] indicated that the chemical risk (scaling, spot formation and corrosion) may be predicted and monitored by the application of a mass balance utilizing solid detention time (SDT) and chloride as control parameters.
BEST CARWASH MANAGEMENT PRACTICES
Best Management Practices (BMPs) are activities developed to help carwash operators reduce contaminants discharged to the environment. BMPs are usually based on pollution prevention principle, which emphasizes reducing or eliminating pollutants and toxic materials at their source rather than removing them from a wastewater stream. Preference should be given to management practices highest in the following hierarchy: (a) avoidance or substitution of polluting products or materials, (b) reduction in the use of polluting products or materials, (c) avoidance of generating polluting by-products, (d) reuse and recycling of polluting by-products and (e) treatment of polluting residual by-products [1,2,3,89].
The following BMPs will help carwash operators decrease the amounts of contaminants entering the sewer system, comply with local regulations, improve the efficiency of their operations and reduce the cost: (a) considering environmental issues and buying best available technology, (b) influencing suppliers by requesting and purchasing less toxic, alternative cleaning products and buying from suppliers who accept materials and containers back for recycling, (c) using only biodegradable, water-based cleaners and avoiding the use of halogenated compounds, aromatic hydrocarbons, chlorinated hydrocarbons, petroleum-based cleaners or phenolics. (d) ensuring employees are trained whenever new equipment is installed or new procedures are implemented, (e) ensuring employees are familiar with the hazards associated with the materials they are using and of potential sources of contamination, (f) ensuring employees understand the site layout and drainage systems and use good housekeeping practices and proper reporting procedures, (g) ensuring employees are aware of the spill response plan and are properly trained to carry it out, (h) storing detergents and other cleaning agents in their proper containers with the correct label and ensuring separate storage of incompatible chemicals to prevent cross contamination and chemical reactions and (i) keeping records of training and offering refresher courses periodically [3,4.5,89].
CONCLUSIONS
Carwash stations use large volumes of water and release wastewater containing harmful chemicals into the environment. The type and quantity of cleaning solutions and finish products used, and the soil particles present on the vehicle, have major effects upon the characteristics of the carwash wastewater effluent. Global water resource supplies are worsening and as a result water shortage will affect 2.7 billion people by 2025. Therefore, understanding how much water is used by the carwash industry and the pollution loads of wastewater produced is necessary to ensure adoption of water conservation measures and to design effective wastewater recycling systems.
Professional car washing is divided into several types based on the technology used. These include self-serve car washing, in-bay automatic car washing, conveyor car washing, touch-free (touchless) car washing and hybrid car washing. This study showed that the amount of water used to wash a car depends on the type of washing process, type and efficiency of washing technology and the type of chemical used. Accordingly, the resulting wastewater will have a varying characteristics and pollution loads. The characteristics of the wastewater resulting from carwash operations are pH, temperature, turbidity, electric conductivity, COD and BOD, solids, nutrients heavy metals, oil and grease, hydrocarbons and microbes, all of which have negative impact on human health and aquatic life.
The growing public concerns for water conservation, health and safety of the public water supply and environmental health of streams, rivers and waterways have led to several environmental regulatory structures designed to protect drinking water and watersheds. Also, a variety of prevention measures, including non-structural and structural activities, are now available to address vehicle washing. These measures must consider the nature of the potential source of contamination, purpose, cost, operational and maintenance requirements of the measures, vulnerability of the source waters, public acceptance of the measures, and the acceptable degree of risk reduction by the community.
Water conservation in the carwash means the efficient use of water through water recycling systems. Carwash water recycling is the use of carwash water that is captured, treated and redirected back into the same use. Water reclamation involves treatment of carwash and rinse water. A properly designed carwash operation is connected to a sanitary sewer that carries the wash water to a biological wastewater treatment plant. However, the environmentally friendly, modern carwash requires a good washing technology, proper water recycling system followed by advanced water treatment methods, and compatible washing chemicals. There are several treatment approaches for managing and recycling wastewater, depending on the size of the site and the resources available. Employees should be aware of operation and maintenance procedures, proper disposal practices, general housekeeping activities and toxic chemicals with which they may come in contact.
Recycling systems reduce or eliminate contaminated discharges to sewer system, storm water drains and water courses. Developments in washing technology provide better quality of wash but also lead to higher water consumption causing the necessity for good car wash reclamation systems. Therefore, with frequently rising water costs, it makes good sense to recycle as much water as possible. Recycling systems leads to higher water quality reused in the washing operation, lower freshwater consumption and reduction or elimination of contaminated discharges to sewer system, storm water drains and water courses. Water conservation should go together with energy conservation in order to make the carwash business efficient.
ACKNOWLEDGEMENTS
The authors appreciate the assistance provided by the Ms. D. M. El Nakib, the Manager of the Bioengineering Laboratory of the Department of Agricultural Engineering, Faculty of Agriculture, Cairo University.
COMPETING INTERESTS
The authors have declared that no competing interests exist.
AUTHORS’ CONTRIBUTIONS
This work was carried out in collaboration among all authors. All authors read and approved the final manuscript.
- Barnes LL (2016) Best management practices for commercial car washes. Great Lakes Pollution Prevention Round Table, United States Environmental Protection Agency, Chicago, Illinois, USA. Available online at: https://www.ideals.illinois.edu/bitstream/handle/2142/98573/BMPs-commercial-car-washes.pdf?sequence=2&isAllowed=y
- Department of Ecology (2012) Vehicle and equipment wastewater discharge: Best management practices manual. Publication Number WQ-R-95-056, Department of Ecology, Olympia, State of Washington, USA. Available online at: https://apps.ecology.wa.gov/publications/documents/95056.pdf
- USEPA (1998) National water quality inventory: Report to congress No. EPA 305(b). United States Environmental Protection Agency, Washington, DC, USA. Available online at: https://www.epa.gov/sites/production/files/2015-09/documents/1998_national_water_quality_inventory_report_to_congress.pdf
- RSCP (2007) Environmental regulations and best management practices. Regional Source Control Program, Environmental Services, Capital Regional District, Victoria, British Columbia, Canada.
- SEPA (2007) Pollution prevention guidelines: Vehicle washing and cleaning. Scottish Environmental Protection Agency, Glasgow, United Kingdom. Available online at: https://www.exeter.ac.uk/media/universityofexeter/campusservices/sustainability/pdf/PPG13_Vehicle_Washing.pdf
- SAEPA (2016) Storm water management for wash bays. Environmental Protection Authority-South Australia, Adelaide, Australia. Available online at: https://www.epa.sa.gov.au/files/7593_water_wash.pdf
- Zaneti RN, Etchepare R, Rubio J (2013) Carwash wastewater treatment and water reuse: A case study. Water Sci Technol 67(1): 82-88.
- Odeyemi OE, Adedeji AA, Odeyemi OJ (2018) Effects of discharge from carwash on the physico-chemical parameters and zooplanktonic abundance of Odo-Ebo River, Ile-Ife, Nigeria. Agric Environ 10: 83-96.
- Aikins S, Boakye DO (2015) Carwash wastewater characterization and effect on surface water quality: A case study of washing bays sited on Oda and Daban streams in Kumasi, Ghana. ARPN J Sci Technol 5(4): 190-197.
- Morel A, Diener S (2006) Greywater management in low and middle-income countries: Review of different treatment systems for households or neighborhoods. Sandec Report No. 14/06, Department of Water and Sanitation in Developing Countries, Swiss Federal Institute of Aquatic Science and Technology, Zurich, Germany. Available online at: https://www.susana.org/_resources/documents/default/2-947-en-greywater-management-2006.pdf
- Brown C (2002) Water use in the professional carwash industry. International Carwash Association. Chicago, Illinois, USA. Available online at: https://www.carwash.org/docs/default-document-library/Water-Use-in-the-Professional-Car-Wash-Industry.pdf
- Williams E (2003) Drying systems. Auto Laundry News, 4(1): 20. Available online at: http://www.carwashmag.com/pdf/april_2003/showcase.cfm
- Janik H, Kupiec A (2007) Trends in modern car washing. J Environ Stud 16(6): 927-931.
- Riddle J (2003) Carwashes put earth first: Businesses, reduce, reuse recycle. Mod Car Care 5: 1.
- Russo S (2003) Can geothermal heating lower your wash’s utility costs? Professional Car Washing and Detailing 5: 1.
- Carwash News (2021) Wind turbine will save energy, educate customers. Carwash News, Carwash.Com. Accessed on: January 20, 2021. Available online at: https://www.carwash.com/wind-turbine-will-save-energy-educate-customers/
- EIC (2010) Variable speed drives for carwash vacuum systems. ET 08.15 Report, Design and Engineering Services, Southern California Edison, Edison International Company, Washington, DC, USA.
- Airlift Doors (2021) Pneumatic carwash bay doors. Airlift Doors Inc, Maple Lake, Minnesota, USA.x
- Utekar P, Naik S, Wadekar M, Watve SG (2015) Implementation of autocar washing system using two robotic arms. Int J Innov Res Sci Eng Technol 4(4): 2546-2551.
- Saber SMM, Marques J, Torres R, Nova N, Mobrega JM (2016) Design of a drying system for a rollover carwash machine using CFD. J Comput Des Eng 3(4): 298-314.
- Tejas B, Varsha B, Manoj K, Ketan K, Kotkar PD (2017) Automatic car washing and drying system. Int J Sci Res Dev 5(2): 2321-0613.
- Subramanian G, Raja KT, Babu KSG, Devashema T (2015) Simulation of automatic car washing using programmable logic controller (PLC). Int J Sci Res Dev 3(1): 2321-0613.
- Mhaske DA, Bhavthankar RG, Darade DJ (2016) PLC based car washing system. Int J Innov Res Electric Electronic Instrumentation Control Eng 4(4): 77-81.
- Hossein A, Sorkhabi D, Khazini B (2013) Manufacturing of fully automatic carwash using intelligent control algorithms. Int J Ind Manuf Eng 7(3): 512-515.
- Vidyasagar K, Ramprasad R, Nagasekhar P (2017) RFID-GSM autonomous car washing system. Int J Comput Appl 121(2): 30-33.
- Shifflett A (2021) Five factors of carwash cleaning. Operation and Management, Carwash News, Carwash.com. Accessed on: March 11, 2021. Available online at: https://www.carwash.com/5-factors-carwash-cleaning/
- Monney I, Donkor EA, Buamah A (2020) Clean vehicles, polluted waters: Empirical estimates of water consumption and pollution loads of the carwash industry. Heliyon 6(5): e03952.
- Fall C, Lopez-Vazquez CM, Jimenez-Moleon MC, Ba KM, Diaz-Delgado C, et al. (2007) Carwash wastewaters: Characteristics, volumes and treatability by gravity oil separation. Rev Mex De Ing Quim 6(2): 175-184.
- Phipps D, Alkhaddar RM, Stiller M (2013) Water saving in domestic car washing. World Environmental and Water Resources Congress: Showing the future (Patterson, C., S. D. Struck and D. J. Murray, Editors) Cincinnati, Ohio, USA. Accessed on: December 20, 2020. Available online at: https://www.researchgate.net/publication/269127543_Water_Saving_in_Domestic_Car_Washing
- Sarne VB (2019) Just how much water does a single car wash use in San Diego, California? VISOR Car News, San Diego, California, USA. Accessed on: December 10, 2020. Available online at: https://visor.ph/industry/just-how-much-water-does-a-single-car-wash-use/
- Lau WJ, Ismail AF, Firdaus S (2013) Car wash industry in Malaysia: Treatment of car wash effluent using ultrafiltration and nanofiltration membranes. Sep Purif Technol 104(1): 26-31.
- USEPA (2008) Managing vehicle washing to prevent contamination of drinking water. Water Protection Practices Bulletin No. EPA816-F-01-024, Office of Water, United States Environmental Protection Agency, Washington, DC, USA. Available online at: https://www.oregon.gov/deq/FilterDocs/EPASWPPracticesBulletin_VehicleWashing.pdf
- Hashim NH, Zayadi N (2016) Pollutant’s characterization of carwash wastewater. MATEC Web Conf 47: 05008.
- Brown C (2002) Water effluent and solid waste characteristics in the professional car wash industry. Report for International Carwash Association, Washington DC, USA. Available online at: https://www.mcacarwash.org/assets/documents/watereffluentsolidwastecharacteristics.pdf
- Czuba JA, Magirl CS, Czuba CR, Grossman EE, Curran CA, et al. (2011) Comparability of suspended-sediment concentration and total suspended solids data and sediment load from major rivers into Puget Sound and its adjacent waters. USGS Fact Sheet 2011-3083, Geological Survey, Tacoma, Washington, USA. Accessed on: May 30, 2020. Available online at: https://pubs.er.usgs.gov/publication/fs20113083
- Hickin EJ (1995) River geomorphology. Wiley and Son Publisher, Chichester, New York, USA.
- Gorsuch AM, Denit J, Stigall CE, Martin EE (1982) Guidance document for effluent discharges from the auto and other laundries point source category. Effluent Guidelines Division, Office of Water and Wastewater Management, United States Environmental Protection Agency, Washington, DC, USA. Accessed on: December 11, 2020. Available online at: https://nepis.epa.gov/Exe/ZyPDF.cgi/9101TK5O.PDF?Dockey=9101TK5O.PDF
- Nguegang B, Sibanda T, Tekere M (2019) Cultivable bacterial diversity, physiochemical profile and toxicity determination of carwash effluents. Environ Monit Assess 191(8): 478-487.
- Sibanda T, Selvarajan R, Tekere M (2019) Targeted 165 rRNA amplicon analysis reveals the diversity of bacterial communities in carwash effluents. Int Microbiol 22(2): 181-189.
- Danha C, Utete B, Soropa G, Rufasha SB (2014) Potential impact of wash bay effluent on the water quality of a subtropical river. J Water Resour Prot 6: 1045-1050.
- EMA (2011) Effluent standards for streams. EMA Environmental Bulletin, Environmental Management Agency, Chikomba District, Harare Province Publications, Harare, Zimbabwe.
- WHO (2000) Guidelines for the safe use of wastewater, excreta and greywater, Volume 2: wastewater use in agriculture. World Health Organization, Geneva, Switzerland. Accessed on: October 12, 2020. Available online at: https://www.who.int/water_sanitation_health/publications/gsuweg2/en/
- Rai R, Sharma S, Gurung DB, Sitaula BK, Shah RDT (2019) Assessing the impacts of vehicle wash wastewater on surface water quality through physico-chemical and benthic macroinvertebrates analyses. Water Sci 40(1): 39-49.
- Chukwu O, Segi S, Adeoye PA (2008) Effect of carwash effluent on the quality of receiving stream. J Eng Appl Sci 3: 607-610.
- Berezina NA (2001) Influence of ambient pH on freshwater invertebrates under experimental conditions. Russ J Ecol 32: 343-351.
- Koryak M, Shapiro MA, Sykora JL (1972) Riffle zoobenthos in streams receiving acid mine drainage. Water Res 6(10): 1239-1246.
- Warner RW (1971) Distribution of biota in a stream polluted by acid mine drainage. Ohio J Sci 71(4): 202-215.
- Tchobanglous G, Burton FL (1991) Wastewater Engineering: Treatment, disposal and reuse. McGraw-Hill Publishing Company, London, United Kingdom.
- Baddor IM, Farhoud N, Abdel-Magid IM, Alshami S, Ahmad FH, et al. (2014) Study of carwash wastewater treatment by adsorption. A paper presented at the International Conference of Engineering, Information Technology and Science, Infrastructure University Kuala Lumpur, Selangor Darul Ehsan, Malaysia. Accessed on: August 20, 2020. Available online at: https://www.academia.edu/22502156/Study_of_Car_Wash_Wastewater_Treatment_by_Adsorption
- Abdel-Magid IM, Olabi A (2014) Study of carwash wastewater treatment by adsorption. International Conference of Engineering, Information Technology, and Science (ICEITS 2014), Infrastructure University Kuala Lumpur, Selangor Darul Ehsan, Malaysia. Accessed on: February 10, 2021. Available online at: https://www.researchgate.net/publication/271704061_Study_of_Car_Wash_Wastewater_Treatment_by_Adsorption
- El-Ashtoukhy EZ, Amin NK, Fouad YO (2015) Treatment of real wastewater produced from Mobil carwash station using electrocoagulation technique. Environ Monit Assess 187(10): 628-638.
- Tekere M, Sibanda T, Maphangwa KW (2016) An assessment of the physico-chemical properties and toxicity potential of carwash effluents from professional carwash outlets in Gauteng Province, South Africa. Environ Sci Pollut Res Int 23(12): 11876-11884.
- Rai R, Sharma S, Gurung DB, Sitaula BK, Raut N (2018) Assessment of environmental impacts of vehicle wash centres at Olakha, Thimphu Bhutan. Int Res J Environ Sci 7: 1-10.
- MWPA (2008) Turbidity: Description, impact on water quality, sources, measures - A general overview. Water Quality-Impaired Waters Bulletin Number #3.21, Minnesota Pollution Control Agency, St. Paul, Minnesota, USA. Accessed on: August 11, 2020. Available online at: https://www.pca.state.mn.us/sites/default/files/wq-iw3-21.pdf
- Al-Gheethi AA, Mohamed RMSR, Rahman MAA, Johari MR, Kassim AHM (2016) Treatment of wastewater from carwashes using natural coagulation and filtration system. Mater Sci Eng 136: 1-7.
- Mohammadi MJ, Salaric J, Takdastand A, Farhadic M, Javanmardie P, et al. (2017) Removal of turbidity and organic matter from carwash wastewater by electrocoagulation process. Desalin Water Treat 68: 122-128.
- Marcos VS (2007) Basic principle of wastewater treatments. IWA Publisher, New York, New York, USA. Available online at: https://www.iwapublishing.com/sites/default/files/ebooks/9781780402093.pdf
- Wiesmann U, Choi I, Dombrowsk EM (2007) Fundamentals of biological wastewater treatment. Wiley Publisher, Hoboken, New Jersey, USA.
- DPW (2012) Evaluation of pollutants in wastewater generated by Mobil commercial car washing operations in Durham, North Carolina. Water Quality Report # 11-001, Department of Public Works, City of Durham, North Carolina, USA. Accessed on: February 5, 2021. Available online at: http://www.ci.bothell.wa.us/DocumentCenter/View/1270/Mobile-Car-Wash-Study-Final-Report-PDF
- Malinen E, Id N, Valtonen S, Hakala J, Mononen T, et al. (2012) Biological treatment of carwash wastewaters - A reduction survey. Article 494 presented at Linnaeus ECO-TECH Conference, Kalmar, Sweden.
- Sprynskyy M, Kosobucki P, Kowalkowski T, Buszewsk B (2007) Influence of clinoptilolite rock on chemical speciation of selected heavy metals in sewage sludge. J Hazard Mater 149: 310-316.
- Jiwan S, Ajay K (2011) Effects of heavy metals on soil, plants, human health and aquatic life. J Res Chem Environ 1(2): 15-21.
- Peng K, Luo C, Luo L, Li X, Shena Z (2008) Bioaccumulation of heavy metals by the aquatic plants Potamogeton pectinatus L. and Potamogeton malaianus Miq. and their potential use for contamination indicators in wastewater treatment. Sci Total Environ 392: 22-29.
- Sobha K, Poornima A, Harini P, Veeraiah K (2007) A study on biochemical changes in the freshwater fish, catla catla (hamilton) exposed to the heavy metal toxicant cadmium chloride. Kathmandu University J Sci Eng Technol 1(4): 1-11.
- Abagale FK, Sarpong DA, Ojediran JO, Osei-Agyemang R, Shaibu AG, et al. (2013) Heavy metal concentration in wastewater from car washing bays used for agriculture in the tamale metropolis, Ghana. Int J Cur Res 5(6): 1571-1576.
- Qu X, Wu N, Tang T, Cai Q, Park YS (2010) Effects of heavy metals on benthic macroinvertebrate communities in high mountain streams. Int J Limnol 46: 291-302.
- Tao Y, Yuan Z, Xiaona H, Wei M (2012) Distribution and bioaccumulation of heavy metals in aquatic organisms of different trophic levels and potential health risk assessment from Taihu lake, China. Ecotoxicol Environ Saf 81: 55-64.
- Eljaiek-Urzola M, Romero-Sierra N, Segrera-Cabarcas L, Valdelamar-Martínez D, Quiñones-Bolaños E (2019) Oil and grease as a water quality index parameter for the conservation of marine biota. Water 11: 856-876.
- Jovcic B, Jebegovic J, Lozo J, Topisirovic L, Kojic M (2009) Dynamic of sodium dodecyl sulfate utilization and antibiotic susceptibility of strain Pseudomonas ATCC19151. Arch Biol Sci 1: 159-164.
- Shabbazi R, Kasra-kemashahi R, Gharavi S, Moosavi-Nejad Z, Borzooee F (2013) Screening of SDS-degrading bacteria from carwash wastewater and study of the alkylsulfatase enzyme activity. Iran J Microbiol 5(2): 153-158.
- Perkowski J, Bzdon S, Bulska A, Jóźwiak WK (2006) Decomposition of detergents present in carwash sewage by titania photo-assisted oxidation. Pol J Environ Stud 15(3): 457-465.
- Jovcic B, Venturi V, Davison J, Topisirovic L, Kojic M (2010) Regulation of sdsA alkyl sulphatase of Pseudomonas sp. ATCC19151 and its involvement in degradation of anionic surfactants. J Appl Microbiol 109: 1076-1083.
- Eichhorn P, Rodriguez SV, Baumann W, Knepper T (2001) Incomplete degradation of SDS in Brazilian surface waters and pursuit of their polar metabolites in drinking waters. Environ Sci 284: 123-134.
- Guangming Z, Haiyan F, Hua Z, Xingzhong Y, Muxing F, et al. (2007) Co-degradation with glucose of four surfactants, CTAB, Triton X-100, SDS and Rhamnolipid, in liquid culture media and compost matrix. Biodegradation 18: 303-310.
- Hosseini F, Amirmozafari N, Malekzadeh F, Ghaemi N (2007) Biodegradation of anionic surfactants by isolated bacteria from activated sludge. Int J Environ Sci Technol 4(1): 127-132.
- Schleheck D, Lechner M, Svhonenberger R, Marc J-F S, Cook AM (2003) Desulfonation of the disulfodiphenylethercarboxylates from linear alkyldiphenyletherdisulfonate surfactants. Appl Environ Microbiol 69: 938-944.
- Scott MJ, Jones MN (2000) The biodegradation of surfactants in the environment. Biochem Biophys Acta 1508: 235-251.
- Bakacs ME, Yergeau SE, Obropta CC (2013) Assessment of carwash runoff treatment using bioretention mesocosms. J Environ Eng 139(8): 1132-1136.
- Linclau E, Ceulemans J, De Sitter K, Cauwenberg P (2016) Water and detergent recovery from rinsing water in an industrial environment. Water Resour Ind 14: 3-10.
- Asano T, Burton F, Leverenz H, Tsuchibashi R, Tchobanoglous G (2006) Water reuse: Issues, technologies, and applications. Metcalf and Eddy Inc., New York, USA.
- USEPA (2004) Guidelines for water reuse. Report Number EPA-625/R-04e108. United States Environmental Protection Agency for International Development, Washington, DC, USA. Available online at: https://sswm.info/sites/default/files/reference_attachments/30006MKD.pdf
- Jefferson B, Palmer A, Jeffrey P, Stuetz R, Judd S (2004) Grey water characterization and its impact on the selection and operation of technologies for urban reuse. Water Sci Technol 50(2): 157e164.
- Brown C (2000) Water conservation in the professional carwash industry. Report for International Carwash Association, Washington, DC, USA. Available online at: https://www.mcacarwash.org/assets/documents/waterconservation.pdf
- Haas C (1996) Acceptable microbial risk. J Am Water Works Assoc 88(12): 8.
- Huertas E, Salgot M, Hollender J, Weber S, Dott W, et al. (2008) Key objectives for water reuse concepts. Desalination 218(1-3): 120-131.
- Zaneti R, Etchepare R, Rubio J (2011) Carwash wastewater reclamation: Full scale application and upcoming features. Resour Conserv Recycl 55: 953-959.
- Smith R, Mleziva S (2014) Wastewater: Solids retention time control in wastewater treatment. Filtr Sep 51(3): 14-17.
- Heidler J, Halden RU (2008) Meta-analysis of mass balance: Examining chemical fate during wastewater. Environ Sci Technol 42(17): 6324-6332.
- FDEP (2005) Guide to best management practices: For 100% closed-loop recycling systems at vehicles and other equipment wash facilities. Pollution Prevention Program, Industrial Wastewater Section, Florida Department of Environmental Protection, Tallahassee, Florida, USA. Available online at: https://floridadep.gov/sites/default/files/GuideBMPClosed-LoopRecycleSystems.pdf


