3761
Views & Citations2761
Likes & Shares
MATERIALS AND METHODS
Isolation, Identification and Preparation of Spore suspension
Three fungal strains were isolated from soil of garbage dump site of Dhapa, Kolkata by standard plating methods in Potato dextrose agar (PDA) media and identified following standard fungal identification key [11]. Spore suspensions were prepared from 8-day old culture (grown in potato dextrose broth media) following the method Cordeiro [12]. All further experiments were done considering MIC and in close proximity to MIC.
Gamma irradiation
2 ml spore suspensions (5 x 105spores/ml) were taken in small centrifuge tubes for gamma irradiation experiment. Spore suspensions were exposed to 20, 40, 60, 80 and 100 Gray of absorbed doses of gamma (absorbed dose of gamma calculated by Fricke Dosimetry) radiation from a Co60 as gamma source (GC 1200, BRIT).
Metabolic/ lignocellulosic Enzyme activity
After 14 days of shaking incubation the biomass part and some portions of broth were separated for determining uptake potential. Remaining broth was centrifuged at 10,000 rpm for 15 mins to avoid any solid debris. The clarified supernatant was used for enzyme assay. In each case 6 replicates were considered for analysis. The gamma absorbed dose which shows maximum growth (in terms of CFU) under metal stress was taken for enzymatic study.
CMCase assay [EC 3.2.1.4]
CMCase activity was studied according to the method of Denison and Koehn [13].
α- Amylase assay [EC 3.2.1.1]
α- Amylase activity was studied according to the method of Bernfield [14].
RESULT AND DISCUSSION
CMCase and α- amylase are two important metabolic enzymes for fungal growth and reproduction as well as responsible for lignocellulose waste degradation. Results of the present study showed the potential of gamma radiation in stimulating activities of these two fungal enzymes (CMCase and α- amylase) grown under metal stress, while metal stress caused decrease in those enzyme activities. Significant enhancement (p≤0.05) of activity of CMCase and α- amylase in gamma irradiated A. Terreus, A.niger and P.cyclopium also noted as a function of increase in absorbed dose of gamma (20-100Gy).Gamma induced effect on α -amylase activity was noted to be more than that noted in case of CMCase in case of A.terreus and P.cyclopium. For A.niger the trend is just opposite i.e. gamma caused more potential enhancement of CMCase activity than α -amylase. Gamma exposed fungal groups showed dose dependent enhancement of both the enzyme activities against every tested fungal strains. In every case maximum escalation was obtained when the fungal strains were exposed at 100Gy of gamma absorbed dose. While a 100Gy exposed group of A.terreus showed 1.13 fold increase in activity of α-amylase, the same dose showed 48% increase in activity of CMCase, when both compared to the un-irradiated normal control counterparts (Figure 1a). Similarly a 100Gy exposed A.niger showed 83% increase in α-amylase activity, while the same group showed 1.08 fold increase in CMCase activity when both are compared with their non-irradiated counterparts (Figure 1b). Parallel to this observations 100Gy exposed P.cyclopium showed 40% more CMCase activity but 2.03fold α-amylase activity with respect to their unirradiated normal control counterparts ( Figure 1c).
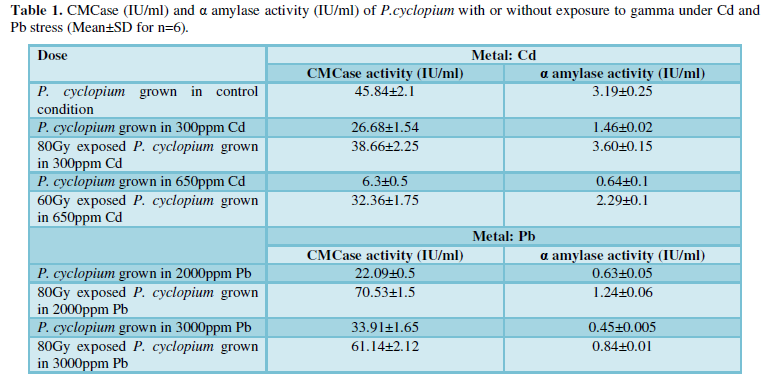
Gamma irradiated groups of P. cyclopium grown in 300-650 ppm of Cd in the media, showed a radiation dose dependent enhancement of CFU up to a certain dose of gamma exposure and the absorbed dose of gamma that produced maximum enhancement in CFU was dependent on concentration of Cd treatment. 80Gy & 60Gy exposed P.cyclopium showed maximum CFU against 300ppm and 650ppm Cd respectively. Similarly 80Gy exposed P.cyclopium showed maximum CFU when grown under Pb stress (2000 & 3000ppm).
P.cyclopium grown in 300ppm Cd showed 57% decrease in CMCase activity and 54% decrease in α -amylase activity. Further increase in Cd concentration i.e from 300ppm to 650ppm, enzyme activities were found to be more declined (86% CMCase and 80% α -amylase) with respect to its control counterparts (Table 1). 80Gy exposed P.cyclopium manifested 95% more CMCase and 1.15 fold more α -amylase activity when grown in 300ppm of Cd with respect to its unirradiated but Cd stressed counterparts. Parallel to this against 650ppm of Cd in growth medium, 60Gy exposed P.cyclopium exhibit 3.56 fold CMCase and 1.14 fold more α -amylase activity when compared to their unirradiated but Cd stressed (650ppm) counterparts. Potential of gamma irradiation in enhancing activities of these enzymes have been documented earlier by a host of workers [9,10]. In the present study, boosting up of activity of CMCase and α-amylase, either to near normal values or even higher, as a result of exposure to gamma irradiation preceding metal exposure, might possibly be an attempt of the fungi to cope up with the stress through stimulation of the enzymes that are directly linked with growth and reproduction of fungi [15].
Similarly 2000ppm Pb caused 52% decrease in CMCase and 80% α -amylase activities in P.cyclopium than control counterparts. Further increase in Pb concentration i.e., from 2000ppm to 3000ppm Pb, enzyme activities were showed to be declined more, 69% CMCase and 86% α -amylase activities. While P.cyclopium prior exposed to gamma and then grown in Pb rich media interestingly enhanced its both enzyme activities.80Gy exposed P.cyclopium when grown in 2000-3000ppm Pb enriched media showed 45%- 96% more CMCase activity and 85%-98% more α-amylase activity respectively when compared with their un - irradiated but Pb stressed (2000-3000ppm) counterparts. Figure 1 depicted significant enhancement (p≤0.05) of activity of CMCase and α- amylase in gamma irradiated P.cyclopium as a function of increase in absorbed dose of gamma (20-100Gy).Gamma induced effect on α -amylase activity was noted to be more than that noted in case of CMCase in case P.cyclopium. 100Gy exposed P.cyclopium showed 40% more CMCase activity but 2.03 fold α-amylase activity with respect to their unirradiated normal control counterparts.
Data of the present study establish the potential of gamma irradiation in modulating metal tolerance in fungi. Effect of gamma irradiation on metal tolerance by the selected fungi indicates strain-specific sensitivity and response of the fungi which is dependent on the absorbed dose of gamma exposure and also on concentration of the metal in the medium [16,17]. Such strain specific behavior of microbes towards metals have been reported by a host of researchers [18,19]. The present postulation is complemented by the observed data reflecting significant enhanced activity of CMCase and α- amylase, the two enzymes involved in growth and reproduction of fungi in metal treated samples pre-exposed to gamma irradiation. Potential of gamma irradiation in enhancing activities of these enzymes have been documented earlier by a host of workers.
It is well established that stress in any form - physical (viz. ionizing radiation etc.) or chemical (viz. heavy metals like Cd, Pb, Zn etc.), causes perturbation in the dynamic equilibrium within the living system. In such condition the cells must be able to adjust their physiological processes to reach a homeostasis. Data of the present study show metal (Cd, Pb) induced depletion in activities of CMCase and α-amylase of all fungal strains, the two enzymes involved in nutrient assimilation and subsequent growth of the fungi. This ensures role of metal in distortion of enzyme functioning in the concerned fungi which is possibly due to association of metal with transcriptional as well as translational pathways as postulated by Baldrian [4]. Heavy metals are known to disrupt normal growth and metabolic activity of the exposed organisms [20]. Usually heavy metal induces uncontrolled efflux/influx of electrolytes or other vital ions resulting in disruption of the ionic homeostasis and subsequent deregulation of activities of many enzymes crucial for basic cell metabolism. In parallel to the present observation Huang [21] found inhibition of CMCase activity and cellulose degradation capacity of Phanerochaete chrysosporium under Pb stress. Earlier, Baldrian [20] also reported decrease of ligninolytic activities in fungi under metal stress. Several studies have shown that certain metals like Fe, Hg, Ag, Cd, Pb inhibited the laccase activity [22,23]. Rhizopus microsporus and Penicillium atrovenetum showed enhance production of industrially important enzyme lipase when these were exposed to various low doses of gamma irradiation (20, 40, 60, 80, 100, 120, 140 and 160 Gy) [24]. Gohel [25] treated Pantoea dispera with two physical mutagens namely UV and gamma absorbed dose and one chemical mutagen i.e., EMS to observe the chitinolytic activity. They found that gamma mutant produces better chitinolytic enzyme than UV mutant. These mutants also better producer of chitinolytic enzyme than their wild strain. This research group further revealed that these mutants were better producer of protease and b -1-3 glucans too as compared with their wild type. Abo-State [9] first isolated potent cellulase producers studying their cellulase productivities on wheat straw, wheat bran, rice straw and corn cob. Most potent strain (Aspergillus sp) exposed to different doses of gamma radiation to determine their further enzyme activity. Similarly, Huma [26] developed hyper amylase producing fungus Phialocephala humicola through gamma ray treatment (100 and 140 krad), which have diverse industrial applications. Three mutants (M13, M16 and M24) were selected based on the hyper-production of α-amylases. Three mutants showed greater than two-fold improvement in enzyme production.
In the present study, boosting up of activities of CMCase and α-amylase, either to near normal values or even higher, as a result of exposure to gamma irradiation preceding metal exposure, might possibly be an attempt of the fungi to cope up with the stress through stimulation of the enzymes that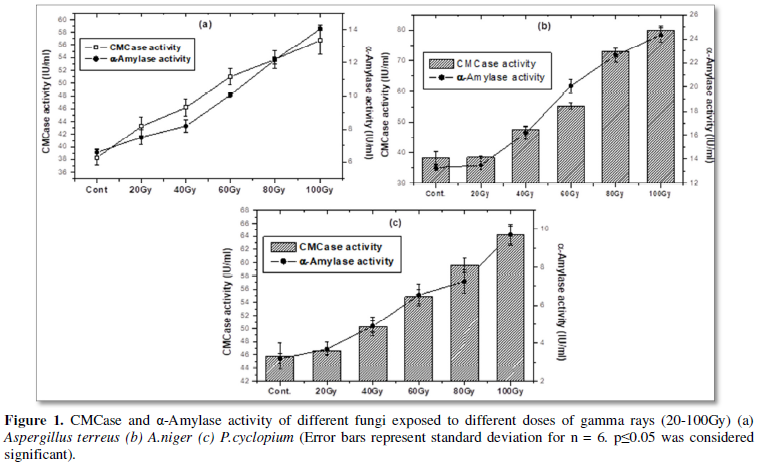
are directly linked with growth and reproduction of fungi [15].
CONCLUSION
Heavy metal stress causes decrease in CMCase and α-amylase activities in tested fungal strains but gamma exposed groups of fungi could capable of enhancing those enzyme activities even under heavy metal stress. This finding shows light to utilize low doses of gamma irradiation for lignocellulosic waste degradation.
-
Kim S, Dale BE (2004) Global potential bioethanol production from wasted crops and crop residues. Biomass Bioenerg 26: 361-375.
-
Kalogo Y, Habibi S, MacLean HL, Joshi SV (2007) Environmental implications of municipal solid waste-derived ethanol.Environ Sci Technol 41: 35-41.
-
Mtui GYS (2009) Recent advances in pretreatment of lignocellulosic wastes and production of value-added products. Afr J Biotechnol 8(8): 1398-1415.
-
Baldrian T, Gabriel J (2003) Lignocellulose degradation by Pleurotus ostreatus in the presence of cadmium. FEMS Microbiol Lett 220(2): 235-240.
-
Zimmermann W (1990) Degradation of lignin by bacteria. J Biotechnol 13(2-3): 119-130.
-
Chand P, Aruna A, Maqsood AM, Rao LVJ (2005) Novel mutation method for increased cellulase production. Appl Microbiol 98(2): 318-323.
-
Li XH, Yang HJ, Roy B, Park EY, Jiang LJ, et al. (2009) Enhanced cellulose production of the Trichoderma viride mutated by microwave and ultraviolet. Microbiol Res 164(1): 81-91.
-
Pradeep MR, Narasimha G (2011) Utilization of Pea Seed Husk as a Substrate for Cellulase Production by Mutant Aspergillus niger. Insight Biotechnol 1(2): 17-22.
-
Abo-State MAM, Hammad AI, Swelim M, Gannam RB (2010) Enhanced Production of Cellulase(S) By Aspergillus Isolated from Agriculture Wastes by Solid State Fermentation. Am-Eur J Agric Environ Sci 8(4): 402-410.
-
Fawzi EM, Hamdy HS (2011) Improvement of carboxymethyl cellulase production from Chaetomium cellulolyticum NRRL 18756 by mutation and optimization of solid-state fermentation. Afr J Microbiol Res 5(26): 4687-4696.
-
Thorn C, Raper KB (1945) A manual of the Aspergilli. Baltimore: Williams and Wilkins.
-
Cordeiro VK, Luna EA, Azevedo JL (1995) Survival and mutant production induced by mutagenic agents in Metarhizium anisopliae. Sci Agric 52: 548-554.
-
Denison DA, Koehn RD (1977) Assay of cellulases. Mycologia. Vol: LXIX, pp: 152.
-
Bernfield P (1955) In; Methods of enzymology (Eds colowick, S and Kaplan, N.O) Academic Press, New York Vol: 1, pp: 149.
-
Tuckwell D, Lavens SE, Birch M, (2006) Two families of extracellular phospholipase C genes are present in aspergilla. Mycol Res 110: 1140-1151.
-
Das D, Chakraborty A, Santra SC (2016a) Effect of gamma radiation on zinc tolerance efficiency of Aspergillus terreus Thorn. Curr Microbiol. 2: 248-258.
-
Das D, Chakraborty A, Santra SC (2016b) Ionising radiation in modulating zinc tolerance potential of Aspergillus niger. Proc Natl Acad Sci India Sect B Biol Sci 86: 39-45.
-
Ahmed I, Ansari M, Aquil F (2005) Biosorption of Ni, Cr and Cd by metal tolerant Aspergillus niger and Penicillium sp using single and multi-metal solution. Indian J Exp Biol 44: 73-76.
-
Iram S, Ahmad I, Javed B, Yaqoob S, Akhtar K, et al. (2009) Fungal tolerance to heavy metals. Pak J Bot 41(5): 2583-2594.
-
Baldrian P, Wiesche C, Gabriel J, Nerud F, Zadrazil F (2000) Influence of cadmium and mercury on activities of ligninolytic enzymes and degradation of polycyclic aromatic hydrocarbons by Pleurotus ostreatus in soil. Appl Environ Microbiol 66: 2471-2478.
-
Huang DL, Zeng GM, Jiang XY, Feng CL, Yu HY, et al. (2006) Bioremediation of Pb-contaminated soil by incubating with Phanerochaete chrysosporium and straw. J Hazard Mater B134: 268-276.
-
Robles A, Lucas R, Martinez-Cañamero M, Omar NB, Pérez R, et al. (2002) Characterization of Lacase Activity Produced by the Hyphomycete Chalara (Syn. Thie-laviopsis) paradoxa Enzyme Microb Technol 31(4): 516-522.
-
Hatvani N, Mécs I (2003) Effects of Certain Heavy Metals on the Growth, Dye Decolorization, and Enzyme Activity of Lentinula edodes. Ecotoxico Environ Safety 55(2): 199-203.
-
Iftikhar T, Mubashirniaz M, Abbas QS, Zia MA, Ashraf I, et al. (2010) Mutation induced enhanced biosynthesis of lipases by Rhizopus oligosporus var microspores. Pak J Bot 42(2): 1235-1249.
-
Gohel V, Vyas P, Chhaptar HS (2005) Activity staining method for chitinase on chitinagar plate through polyacrylamide gel electrophoresis. Afr J Biotechnol 1(1): 87-90.
-
Huma T, Rashid MH, Javed MR, Ashraf A (2012) Gamma ray mediated mutagenesis of Phialocephala humicola: Effect on kinetics and thermodynamics of α-amylase production. Afr J Microbiol Res 6(22): 4639-4646.



