3838
Views & Citations2838
Likes & Shares
The aim of this study was to evaluate the toxicity of the methanol leaf extract of Anacardium occidentale (cashew) Carica papaya (pawpaw) and Psidium guajava (guava) on hepatic and renal functions. Indices of hepatic and renal functions which were measured using standard procedures revealed a non-significant difference in the activities of Aspartate transaminase (AST) of the treated groups (II-IV) compared to the normal control group. However, for Alanine aminotransferase (ALT) (41.67±1.20), (43.67±1.20), (40.33±1.46) and Alkaline phosphatase (ALP) (65.67±1.76), (60.67±6.36), (60.33±5.78) the values recorded for the treated groups were significantly higher compared to the normal control groups (36.67±1.20) and (57.67±43.33) respectively. Measurement of renal function indices revealed that the value of urea for the treated groups (II-IV) (38.67±0.88), (39.00±2.086) and (37.00±2.08) was significantly higher than that of the normal control group (28.67±3.17). However, for total protein and albumin, there was no significant difference in values recorded for the treated groups and the normal control group. In conclusion, findings indicate that pawpaw, guava and cashew leaves extract may contain compounds which may induce serum hepatomakers, which however may not be suggestive of any form of liver damage.
Keywords: Hepatic, Renal, Toxicity, Injuries, Protein
INTRODUCTION
Medicinal plants as the name implies are plants whose part or parts have been recognized for its therapeutic abilities. Plants’ parts such as the leaf, stem bark, and root which have been used both for prophylactic and therapeutic purposes against diseases affecting mankind have been documented in reputable scholarly articles [1]. About 1500 plants are known globally for their therapeutic potentials. The use of medicinal plants in the health care system has been in existence since prehistoric times and has been absorbed into the African cultural systems where plant derived medicinal preparations have been relied upon to treat diverse infectious and non-infectious diseases [2].
In accordance with World Health Organization (WHO) report, about 90% of the populations of developing countries depend on the use of plant-based preparations to address numerous diseases ravaging them [3]. The interest in the use of medicinal plants in the treatment of diseases has been propelled by the characteristic toxic influence of their synthetic counterparts on biological systems [4].
It is a widely held belief, that therapeutic preparations derived from medicinal plants are not toxic. This impression could be misleading as research has established the toxic effects of such preparations on the health of subjects which is dependent on factors such as the strength of secondary metabolite and the quantity of the material consumed [5].
Carica papaya (pawpaw) which is predominantly cultivated in the tropics is a member of the family Caricacae [6]. Research has shown that parts of C. papaya are embodiment of compounds with wide therapeutic potentials.
Notable examples of such compounds are the anti-oxidants and minerals reportedly present in different parts of the plants [7]. Leaf of C. papaya is used in the treatment of diseases such as dyspepsia, hyperacidity, dysentery and constipation etc. [8].
Psidium guajava (guava) is a well-known tropic tree which is mainly grown for its fruit. Belonging to the family myrtaceae, it is cultivated in South Africa [9]. The leaf has been extensively explored in the treatment of numerous human diseases [10]. It is consumed not only as food but also as a folk medicine in sub-tropical areas all over the world due to its pharmacologic activities and has been used as anti-inflammatory and anti-diabetic agent [11].
Anacardium occidentale which can also be referred to as cashew belongs to the family Anacardiaceae [12]. The tree is native to Brazil, although it is found in tropical countries. Different parts of the Anacardium occidentale have been used in the treatment of diverse diseases ravaging mankind. These include bacterial and fungal infection, and oxidative stress related conditions [12]. Although these plants are inherently endowed with therapeutic potentials, the possibility of the presence toxic components cannot be completely ruled off and thus has informed the basis for this research work [13].
MATERIALS AND METHODS
Collection of plant material
Fresh leaves of Cashew (Anacardium occidentale), pawpaw (Carica papaya) and Guava (Psidium guajava) were collected from Zaria metropolis. Identified and authenticated at the Herbarium unit of the Department of Biological Sciences, Ahmadu Bello University, Zaria.
Preparation of plant extracts
Leaves sourced from the three different plants were separately dried at room temperature, after which they were separately ground to powder using an electric blender. The resulting powder was sieved to obtain fine powder. 500 g of the powdered plant sample was separately suspended in 2 Liters of 70% methanol for 72 hours and stirred intermittently. The extracts were filtered and the filtrates concentrated at 400C [14].
Animals
Adult male Wister rats (150-200g) were procured from the animal house of the Department of Pharmacology and Therapeutics, Ahmadu Bello University, Zaria. The animals were housed in well ventilated cages and fed with rat chow and water ad libitum.
Acute toxicity study
The acute toxicity test was performed in accordance with the method of Lorke [15]. The initial phase was characterized by the division of the rats into three (3) groups of three rats per group and were orally administered with 10mg, 100mg and 1000mg of the extracts per kg body weight respectively. They were observed for 24 hours for signs of toxicity. In the absence of which the second phase was initiated and made up of three (3) rats which were divided into three groups of 1 rat per group and administered with 1600, 2900, 5000mg/kg bw of the test sample.
The LD50 was determined from the results of the final phase as the square root of the product of the lowest lethal dose and the highest non-lethal dose.
Experimental Design
Twenty adult male albino rats were divided into four groups of five rats each.
- Group I: Normal control was fed with only rat chow and water ad libitum.
- Group II: Rats were administered with 200 mg/kg b. w of methanol extract of Carica papaya (pawpaw) leaf orally.
- Group III: Rats were administered with 200 mg/kg b.w of methanol extract of Anacardium occidentale leaf orally.
- Group IV: Rats were administered with 200 mg/kg b.w of methanol extract of Psidium guajava (guajava) leaf orally.
Collection of blood sample and preparation of serum
The experiment was terminated on the 28th day of experiment, after which rats were sacrificed and blood samples collected in red topped tubes and allowed to stand at room temperature for 15-30 minutes to clot. Blood samples were centrifuged at 1500 x g for 10 minutes. The serum was transferred into a collecting tube with the aid of a pipette.
Renal Function Test
Serum urea concentration:
Exactly 2ml of water was introduced into test tubes; this was followed by the inclusion of 10µL of test sample into. Into the test tube, 10µL of standard reagent was added into a separate test tube. 2 ml of uric acid color reagent was introduced into all test tubes and incubated for 20minutes. The blank was used to zero the machine. The absorbance of the test samples and the standard were read at 500nm and the concentration of the test sample was determined [16].
Determination of total proteins
Exactly 1 ml of biuret reagent was added into sample test tubes containing 20µl of test sample. Precisely 20µl of the standard reagent was introduced into a separate test tubes were allowed to incubate for 10mins under room temperature. The blank was used to zero the machine and absorbance was read at 540nm [17].
Determination of albumin concentration
Precisely 1 ml of bromocresol green reagent was introduced into sample test tubes containing 20µl, exactly 20µl of the standard reagent was added into another test tube. The test tubes were incubated for 10min under room temperature. The blank was used to zero the machine and the absorbance was read at 630nm [18].
Evaluation of Markers of Liver Function
Determination of aspartate aminotransferase
Exactly 1 ml of reagent was introduced into sample test tubes containing 500 µl of test sample and 50µl of the standard reagent was added into a separate test tube. The test tubes were incubated at room temperature for 20mins, mixed immediately and first absorbance of test was read exactly at 1minute and thereafter at interval of 30, 60, 90 and 120 seconds at 340nm. The mean change in absorbance was determined [19].
Determination of alanine aminotransferase activity
Precisely 1 ml of reagent was introduced into sample test tubes containing 500 µL of test sample. Exactly 50µl of the standard reagent was added into a separate test tube. Samples were incubated at room temperature for 20mins, it was mixed immediately and first absorbance of test was read at exactly 1minute and thereafter, 30, 60, 90 and 120 seconds at 340nm. The mean change in absorbance per minute was determined [19].
Determination of alkaline phosphatase activities
Precisely 3 ml of substrate solution was incubated at 370C for 15 mins after which 0.5ml of test samples was introduced. The mixture was shaken vigorously. 0.05 ml of the mixture was removed and mixed with 9.5 ml of 0.085 N NaOH. This corresponded to zero-time assay (blank). The remaining solution (substrate+enzyme) was incubated for 15min at 370C and then 0.5 ml was drawn and mixed with 9.5ml of 0.085N NaOH. Absorbance was measured at 405 nm against the reference blank [20].
Statistical Analysis
Results were expressed as mean ± standard error of mean (SEM). The data was analyzed with the aid of the analysis of variance (ANOVA). The differences in mean among groups were compared using the Duncan Multiple Range Test. P value less than 0.05 was considered significant.
RESULTS AND DISCUSSION
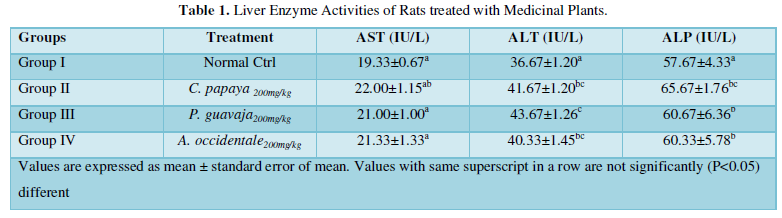
Medicinal plants can be deleterious to human health. This is evident by the fact that about some plants have been reported to contain toxic components which are mainly secondary metabolites [21]. Table 1 shows the activity of liver enzymes of experimental rat models sorted into groups and administered with 200mg/kg b.w methanol leaf extract of Anacardium occidentale (cashew) Carica papaya (pawpaw) and Psidium guajava (guava) leaves accordingly. There was no significant difference in the activity of aspartate aminotransaminase (AST) of treated rats i.e., Groups II-IV compared to that of the untreated control group I. This may be attributed to the nature or strength of the inherent toxic components, the dose of extract administered, or even the part of the plants ingested [5]. This result is consistent with the finding of Nwiloh which infers that the aqueous leaf extract of the aforementioned plants did not induce significant increase in the activity of aspartate aminotransferase (AST).
However, there was a significant increase in the activities of ALT and ALP of treated groups compared to the control. This result is consistent with the sub-acute toxicity study performed by Charlse [22] which affirms that oral administration of (10-500 mg/kg) methanol leaf extract of the plants being studied for 14 days increased the activities of serum hepatomarkers.
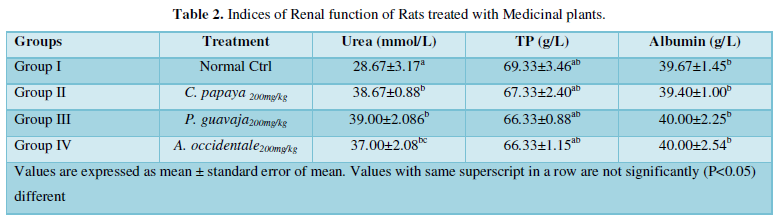
Table 2 shows the renal indices of experimental rat models drafted into groups and treated with methanol leaf extract of Anacardium occidentale (cashew) Carica papaya (pawpaw) and Psidium guajava (guava) leaves. The concentration of urea was significantly (P>0.05) high in treated groups compared to the normal control. On the other hand, there was no significant (P<0.05) difference in the concentration of total protein and albumin in the treated groups compared to normal control group.
This may be as a result as of the nature the secondary metabolites, the dose of extract administered, or even the part of the plants consumed [5]. This result is consistent with the finding of Halim [22] which shows that urea concentration increased in rat models administered with leaf extract of cashew, pawpaw and guava.
CONCLUSION
Findings from this work indicate that there may be inherent compounds capable of inducing liver enzyme which may not necessarily imply a damaged liver.
- Gurib-Fakim A (2006) Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol Aspects Med 27: 1-93.
- Diallo D, Paulsen BS, Liljebäck TH, Michaelsen TE (2003) The Malian medicinal plant Trichilia emetica, studies on polysaccharides with momplement fixing ability. J Ethnopharmacol 84: 279-287.
- van Andel T, Carvalheiro LG (2013) Why urban citizens in developing countries use traditional medicines: the case of Suriname. Evid-Based Compl Alt Med 2013: 687197.
- Mbi CW, Bilikha JB (1998) Conventional drug production from medicinal plants with contribution from the Cameroon Pharmacopoeia. Herbal 98 abstracts, Ibadan, Nigeria. pp: 13-14.
- Fall M, Boukandou M, Fall AD, Cabral M, Diatta W, et al. (2011) Toxicité aiguë et subaiguë d’extrait aqueux de feuilles d’Aphania senegalensis (Juss. ex Poir.) sur des rats Wistar. Dakar Med 56: 216.
- Tülay AC (2012) Potential genotoxic and cytotoxic effects of plant extracts; A compendium of essays on alternative therapy. Edited by Arup Bhattacharya. pp: 233-250. Available online at: https://www.intechopen.com/books/a-compendium-of-essays-on-alternative-therapy/potential-genotoxic-and-cytotoxic-effects-of-plant-extracts
- Gill LS (1992) Ethnomedical uses of plants in Nigeria. 2nd Nigeria Uniben Press. Benin.
- Kenneth S, Brekke L, Johon E, Donald S (1970) Volatile constituents in guava. J Agri Food Chem 18: 598-599.
- Grover JK, Yadav S, Vats V (2002) Medicinal plants of India with antidiabetic potential. J Ethnopharmacol 81: 81-100.
- Deguchi Y, Miyazaki K (2010) Anti-hyperglycemic and anti-hyperlipidemic effects of guava leaf extract. Nutr Metab 7(9): 1-10.
- Dhananjay D, Chaitanya R, Suresh P, Rajashri G, Dattaguru P (2017) Anacardium occidentale: Fountain of phytochemicals; The qualitative profiling. World J Pharm 6(5): 585-592.
- Nasri H, Hedayatollah S (2013) Toxicity and Safety of Medicinal Plants. J Herb Med Pharmacol 2(2): 21-22.
- Phrompittayarat W, Putalun W, Tanaka H, Jetiyanon K, Wittaya-areekul S, et al. (2007) Comparison of various extraction methods of Bacopa monnier. Naresuan Uni J 15(1): 29-34.
- Lorke D (1983) A New Approach to practical acute toxicity testing. Arch Toxicol 54(4): 275-287.
- Fawcett JK, Scout JE (1960) A rapid and precise method for the determination of urea. J Clin Pathol 13: 156-159.
- Bode C, Goebel lH, Stahler E (1968) The elimination of errors caused by turbidity in the determination of protein by the biuret method. Z Klin Chem Klin Biochem 6: 418-422.
- Autenrieth W (1917) Colorimetric estimation of serum albumin and globulin in urine, ascetic fluid and blood serum. Munch, med. Wochenschr 64: 241-245.
- Amador E, Wacker WEC (1962) Serum glutamic-oxaloacetic transaminase activity: A new modification and an analytical assessment of current assay technics. Clin Chem 8(4): 343-350.
- Haussament TU (1977) Qualitative determination of serum alkaline phosphate. Clin Chim Acta 35(27): 1-273.
- Ishii R, Yoshikawa H, Minakata NT, Komura K, Kada T (1984) Specificity of bio antimutagens in the plant kingdom. Agric Biol Chem 48: 2587-2591.
- Charles A, Jemima A, Appiah K, Boadu M, Priscilla KM (2016) Aqueous leaf extract of Carica Papaya (Caricaceae) Linn. causes liver injury and reduced fertility in rats. Int J Pharm Pharm Sci 8(2): 6-76.
- Halim SZ, Abdullah NR, Afzan A, Rashid BAA, Jantan I, et al. (2011) Acute toxicity study of Carica papaya leaf extract in Sprague Dawley rats. J Med Plants Res (8): 1867-1872.


