2818
Views & Citations1818
Likes & Shares
Human repair-neurophysiology is a new discipline with which the human nervous system can be repaired. Since the nervous system is involved in nearly all body functions, the general health can be improved. By combining Human Neurophysiology with the System Theory of Pattern Formation, there is a theoretical basis that through movement-based learning vegetative and cognitive functions can also be repaired by learning transfer. Bladder continence is achieved via the repair of the vegetative (inner bladder sphincter) and the somatic nervous system (external bladder sphincter) and their coordination. Bladder dyssynergia can be repaired.
Based on human repair-neurophysiology [1,2], a movement-based learning therapy was developed through neural network learning [3], called Coordination Dynamics Therapy (CDT), with which it is possible to improve or repair central nervous system (CNS) functioning after stroke [4], traumatic brain injury [5,6], spinal cord injury [7-13], cerebellar injury/atrophy [14,15], cerebral palsy [16], hypoxic brain injury [17], in Parkinson’s disease [18], spina bifida (myelomeningocele) [19] and scoliosis [20]. Speech had been induced and improved in a patient with severe cerebral palsy [1]. A permanent coma patient could be brought out-of-coma and relearned to speak and move [21-26] and cancer grows could be inhibited through CDT [22,23] by improving cardio-vascular performance [1,21] and building of natural killer (NK) cells [24]. Urinary bladder functions [1] could be cured in cerebral palsy [1] and spinal cord injury [7,12,13]. There is indication that general health can be improved via CDT to live longer with a better quality of life [25] and euthanasia can be avoided in organ donation [26]. Basal ganglia injury was also repaired [27] and spinal muscular atrophy stopped [28].
Recording of single-nerve fiber action potentials
The progress in repairing the human sacral micturition center started with a new recording technique, the single-nerve fiber action potential recording method [29,30].
The development of the single-nerve fiber action potential recording method was possible because of the unique anatomical landscape of the human spinal canal. Because of the Ascensus of the spinal cord, the lumbosacral nerve roots are very long and form the cauda equina (Figure 1A). Since the caudal sacral nerve roots are very thin and nerve roots are only ensheathed by a thin layer of cells, they are ideal for recording single-nerve fiber action potentials (APs) from undissected nerve roots. Since humans have no tail, continence (mainly S2 to S5) and sexual functions are mainly located in the conus medullaris only. Those functions are therefore represented in the lower sacral nerve roots and they are not intermingled with tail functions as they are for example in rats, cats and dogs.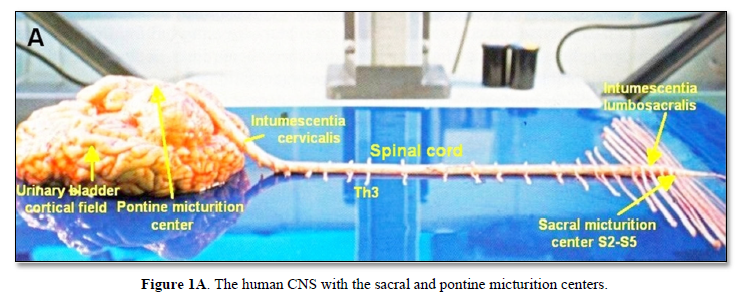
The recording arrangement during an operation is shown in Figure 1B. To obtain natural impulse patterns simultaneously from several single afferent and efferent nerve fibers to analyze CNS functioning, the summed impulse traffic of several afferent and efferent fibers of a nerve root has to be split into the impulse patterns of single fibers. The splitting is achieved by recognizing the APs from certain single fibers on the basis of wave form comparisons on the two recording traces and the conduction time which an AP needs to travel from one electrode pair to the other one (10mm) and selecting these APs out. The summed impulse traffic of the recording is split into the impulse patterns of several single afferent and efferent nerve fibers. In the thin lower sacral nerve roots, there are afferent and efferent fibers in ventral and dorsal roots.
As we can measure the natural impulse patterns, generated by certain single receptors in the periphery, which run into the spinal cord (CNS) (afferents) and those patterns which leave the cord (efferents) in ensembles of single fibers simultaneously, it becomes possible to analyze the integrative properties of the largely unchanged CNS of brain-dead humans (HTs) at a cellular level. This also means that the change in function, caused by CNS injury, can be identified.
Classification of human peripheral nerve fibers by the group conduction velocity and the group nerve fiber diameter
For the analysis of CNS functioning and neural network learning for repair, we must first identify the kind of nerve fibers from which the recordings are taken. A classification scheme of human peripheral nerve fibers is needed.
Conduction velocities of single nerve fibers were therefore calculated from the conduction distance (electrode pair distance = 10 mm) and the conduction times (the time difference of a certain AP between the traces from two pairs of wire electrodes). Velocity distributions of afferent and efferent fibers were constructed, and distribution peaks were correlated to certain nerve fiber groups. The nerve roots from brain dead and surgical patients could be removed, fixated, embedded and stained. Mean nerve fiber diameters could be measured, and nerve fiber diameter distributions constructed for different myelin sheath thicknesses (morphometry). By correlating the identified conduction velocity peaks with nerve fiber diameter peaks (Figure 1B), a classification scheme for the human Peripheral Nervous System (PNS) was constructed, in which individual groups of nerve fibers are characterized by group conduction velocities and group nerve fiber diameters [31-33] (Figure 1C).
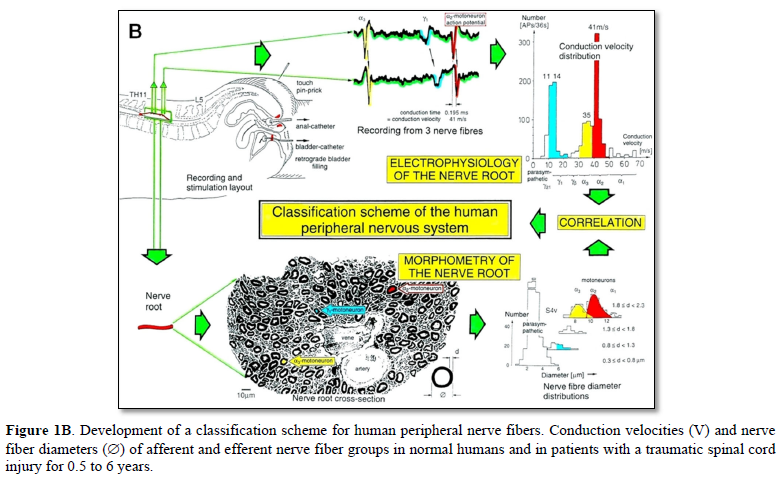
The group conduction velocities and group nerve fiber diameters had the following pair-values at 35.5°C:
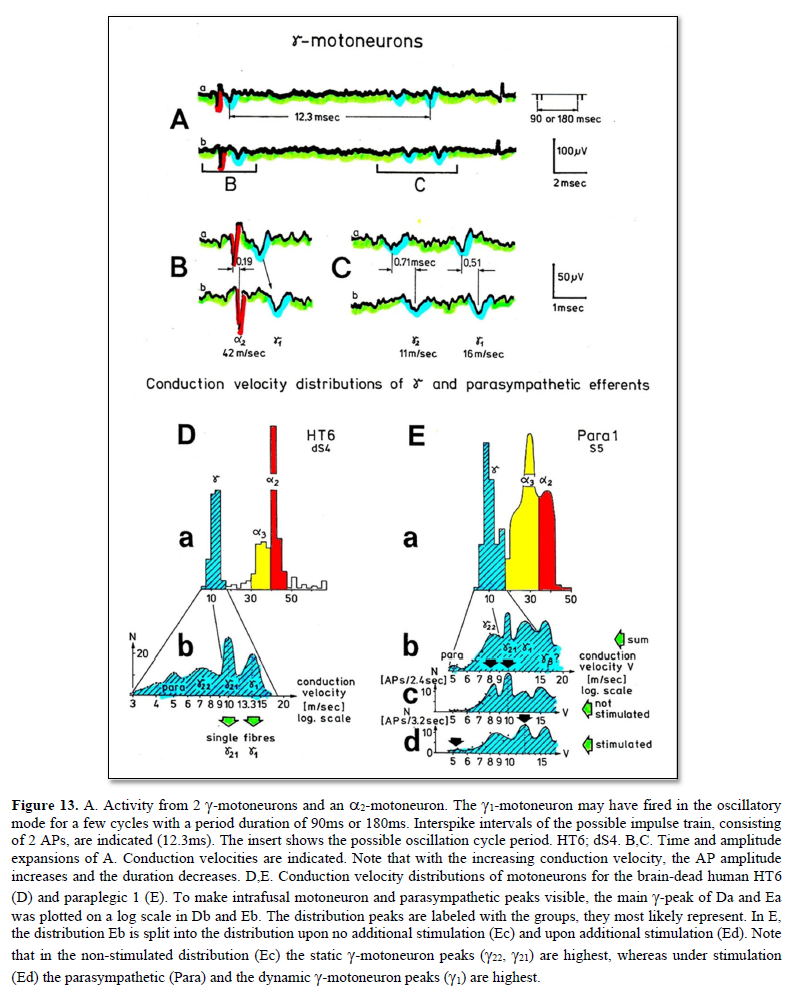
Spindle afferents: SP1(65ms-1/13.1µm), SP2(51/12.1); touch afferents: T1(47/11.1), T2(39/10.1), T3(27/9.1), T4(19/8.1); urinary bladder afferents: S1(41ms-1/-), ST (35/-); a-motoneurons: a13 (-/14.4), a12 (65ms-1/13.1µm), a11 (60?/12.1)[FF], a2 (51/10.3)[FR], a3 (41/8.2)[S]; g-motoneurons: gb(27/7.1), g1(21/6.6), g21(16/5.8), g22(14/5.1); preganglionic parasympathetic motoneurons: (10ms-1/3.7µm).
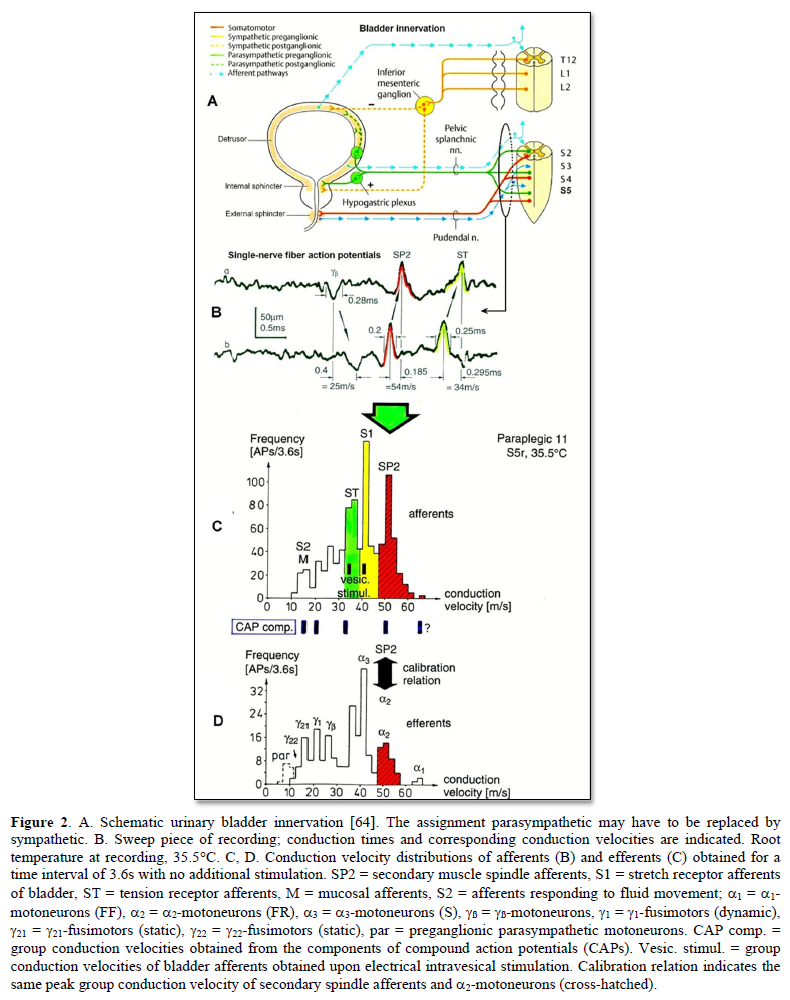
Since the conduction velocity of nerve fibers depends on the temperature, a calibration relation is needed and exists, namely that the secondary muscle spindle afferents (SP2) conduct with the same velocity as the a2-motoneurons independently on the temperature (Figure 2). This classification scheme is still incomplete and holds only for nerve fibers thicker than approx. 3.5µm. The classification schemes for animals do not apply to humans. Conduction velocities in rats, cats and dogs (max » 120 m/s), for example, are much higher than those in humans (max » 70 m/s).
It will thus become possible to record natural impulse patterns simultaneously from identified single afferent and efferent nerve fibers and analyze self-organizing mechanisms of the human CNS under physiologic and pathologic conditions, especially from the human sacral micturition center (Figure 2).
As we can measure simultaneously the natural impulse patterns, generated by certain identified single receptors in the periphery induced by touch, pin-prick, anal and bladder catheter pulling and bladder filling, which simultaneously run into the spinal cord (CNS) and those patterns which leave the cord, it becomes possible to analyze the integrative properties of the largely unchanged CNS of brain-dead humans (HTs) and the injured CNS in an operation of spinal cord injury patients at the cellular level and compare them.
Self-organization of premotor spinal network oscillators
Typical firing patterns of motoneurons can be observed when motoneurons are activated with increasing strength of adequate afferent input. This can be achieved when recording from a motoneuron innervating the external bladder sphincter and filling the bladder (Figure 3). With low afferent input, the motoneurons fire occasionally. With increasing input, they fire intermittently in an oscillatory manner and then continuously oscillatory (Figure 3). The demonstration that neurons of the CNS, in this case motoneurons, can fire with both in an oscillatory and non-oscillatory manner is very important for the understanding of the functioning of the human CNS. To describe the functioning of the CNS merely by reflex pathways and loops or coupling of rigid oscillators (of cellular or network origin) is in contradiction to empirical human data, namely that premotor spinal oscillators self-organize as was concluded from measurements of simultaneous natural impulse patterns of afferent and efferent fibers (Figure 3).
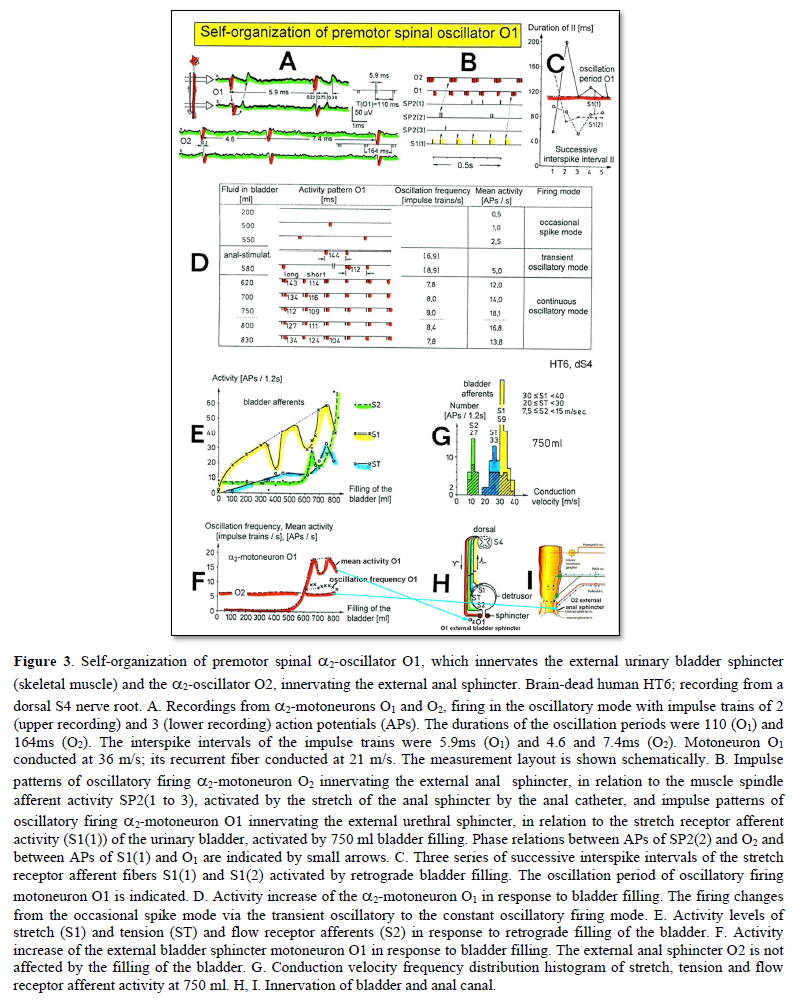
As will be shown below, these self-organized premotor spinal network oscillators, of which the motoneuron is most likely a part, are sub-neural networks which coordinate their functioning. When this coordinated communication becomes impaired due to insufficient inhibition, they synchronize their firing with the consequence of pathologic tremor occurring in patients with Parkinson’s disease [18].
In what follows, I shall concentrate mainly on the oscillatory firing of motoneurons [34-36], which takes place for high activation. In this high activation mode these self-organized network oscillators can also be used as a reference basis when defining phase relations and thus phase and frequency coordination can be measured among neuron firing. For high and rather constant afferent input it was found that a-motoneurons fire repeatedly with impulse trains according to their type. The a1-motoneurons (FF) fire rhythmically at around 10 Hz (range 8 to 20) with an impulse train consisting of 1 AP; a2-motoneurons (FR) fire at approx. 6 to 9 Hz with 2 to 5 APs per impulse train, and a3-motoneurons (S) fire with a frequency in the range of 1 Hz and with long impulse trains consisting of up to 40 APs (and more). The rhythmic firing patterns of a-motoneurons are probably generated by local neuronal networks of the spinal cord since oscillatory firing can be recorded from motoneurons of the disconnected spinal cord. Probably the motoneuron is a part of the spinal network oscillator. The oscillation period (T) is roughly related to the number of action potentials (APs) per impulse train (nAP), and this can be expressed by the formula: T = 70ms + 30ms · nAP. A typical premotor a2-oscillator fires with 3 APs every 160ms (T = 70ms + 30ms · 3 = 160ms), and can change its firing pattern to 2 APs every 130ms for less activation or to 4 APs every 190ms for higher activation (Figures 4 & 5).
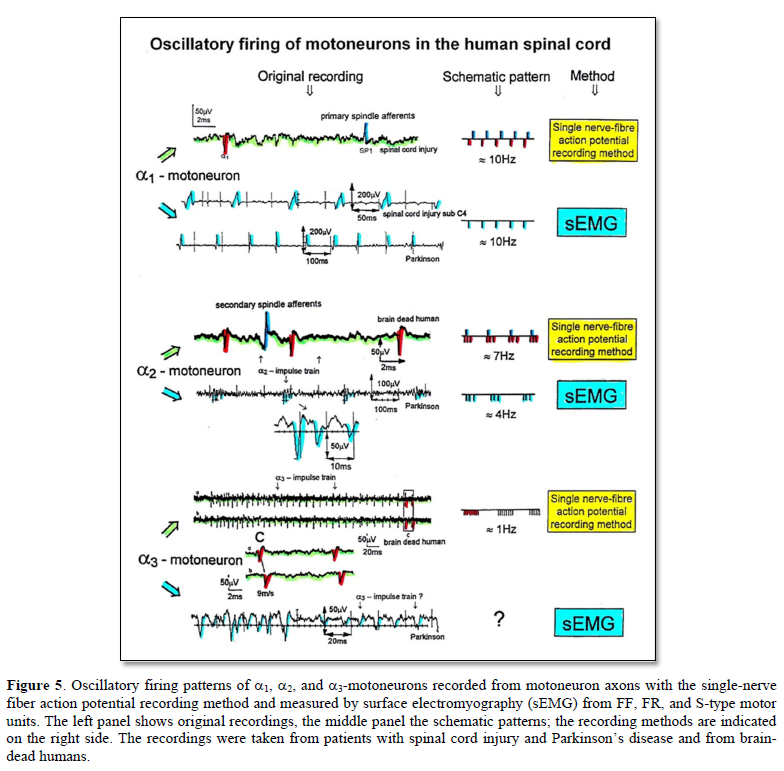
The a1-oscillators respond very dynamically, but have few oscillator network properties. Their firing is absolutely correlated to the firing of primary spindle afferent fibers. The a2-oscillators respond less dynamically, have strong oscillatory properties and self-organize by the adequate afferent input patterns from several kinds of receptors including secondary muscle spindle and urinary bladder afferents. The behavior of a3-motoneurons is more static and their input is polymodal. The dynamics of responding to inputs increases from a3 via a2 to a1-oscillator in accordance with the dynamics of the 3 muscle fiber types the a-motoneurons innervate. The slow (S), medium fast (FR) (fast fatigue-resistant) and fast contracting muscle fibers (FF) (fast fatigable) have their own corresponding premotor networks in the spinal cord, namely that in which the a1, a2 and a3-networks are integrated in.
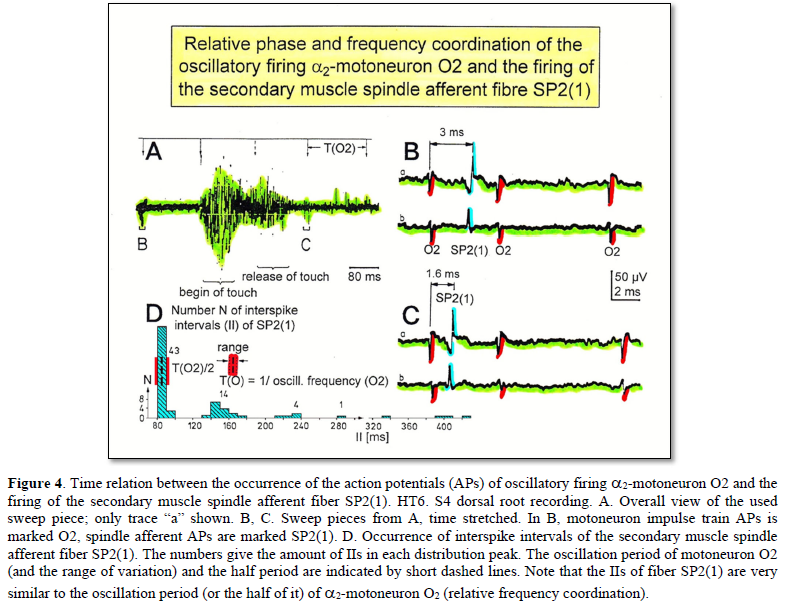
Figure 3 shows an important recording of urinary bladder and rectum functions from the brain-dead human HT6 at the single-neuron level, which will be used below as the healthy comparison when recording from a patient with bladder dysfunction. It is recorded from the premotor spinal a2-oscillator O1 (Figure 3A), which innervates the external urinary bladder sphincter (skeletal muscle) and the a2-motoneurons O2, which innervates the external anal sphincter (Figures 3F,H & I). The external anal sphincter motoneuron O2 fires oscillatory because the anal reflex is stimulated by the anal catheter. The bladder sphincter motoneuron O1 is firing with increasing activity from occasionally to oscillatory when filling the bladder retrogradely. The external bladder sphincter motoneuron starts to fire from 500ml filling onwards to secure continence. The external anal sphincter O2 is nearly not affected by the bladder filling. Simultaneously it is recorded from the bladder afferents, namely the urinary bladder stretch (S1), tension (ST) and flow (S2) receptors (Figures 3E & G). With bladder filling the stretch, tension and flow receptors increase their activity (Figure 3E). Figure 3B indicates schematically that there are phase and frequency relations between the stretch receptor S1(1) and the motoneuron O1 action potentials and between the secondary muscle spindle SP2(2) and the motoneuron O2. Both afferents contribute to the drive of the motoneurons because of the existing phase relations, one is activated by the bladder filling (S1(1)) and the other one (SP2(2)) by the stretch of the anal sphincter caused by the placed anal catheter. Figure 3C shows some real interspike intervals of bladder stretch receptor afferences and the oscillation period of the a2-oscillator O1.
Such good and detailed recording as shown in Figure 3, documented and analyzed at the single-neuron level, is a treasure and gives a detailed survey of the ongoing organizations in the sacral micturition center when communicating with the periphery, namely the bladder and rectum.
Phase and frequency coordination
Now it is tried to measure organization principles of the human CNS. It will be shown that neurons and sub-neuronal networks coordinate their firing up to a few milliseconds. If this phase and frequency [36-38] coordination becomes impaired, organization patterns of the CNS become impaired, instable or are even lost. Every functional or structural modulation of the neuronal networks changes this phase and frequency coordination among neuron firing. Learning is related to the exactness and complexity of the much coordination among single neuron firings or sub-neuronal networks. One strategy for repair is to improve the by injury impaired phase and frequency coordination among neuron firing by movement-based learning. The coordinated movements have to activate the CNS integrative, so that as many phase and frequency coordination’s as possible are trained simultaneously to improve the exactness and complexity of CNS self-organization. By exercising very coordinated movements on the special coordination dynamics therapy (CDT) device, the CNS learns from the device via the movement induced afferent input to improve its coordinated firing of neurons and sub-neural networks.
Relative phase and frequency coordination between the APs of the oscillatory firing a2-motoneuron O2, innervating the external anal sphincter, and the secondary muscle spindle afferent fiber SP2(1) can directly be seen in the original recordings in Figures 4B & C. The firing of the oscillator and the sweep pieces which are shown time-expanded are indicated at the summary trace “D”. Figure 4B, C shows the AP-impulse train of oscillator O2 in connection with one of its driving spindle afferent AP. Because of the duration of the phase relation of around zero milliseconds between the firing of the driving SP2(1)-fiber (firing mostly every 80ms) and the impulse train of the oscillatory firing motoneuron O2 (T(O2) ≈ 160ms), the SP2(1)-fiber AP (every second AP) appeared at a similar time as the impulse train. Because the AP of the spindle afferent fiber had a characteristic waveform, it was easy to extract its impulse pattern from the summed impulse traffic of this S4 dorsal root. During touch-induced skin afferent activity, the activities of the motoneuron and the spindle afferent fiber were covered by the skin afferent activity. After the cessation of the skin afferent activity the afferent and efferent APs were found again at their expected time positions of the regular firings. The phase coordination between the firings of the oscillatory firing motoneuron O2 and the secondary muscle spindle afferent fiber SP2(1) at the time when records B, C were taken, was 1.6ms (3ms - 1.4ms, Figures 4B & C). In Figure 4D, the relative frequency coordination between the firings of the SP2(1)-fiber and the impulse train of the oscillator is indicated. For the time period evaluated, the correlation, the phase and frequency coordination, between the firing of the motoneuron and the spindle afferent fiber was in the range of between 3 and 5ms (Figure 4D).
Similar efferent impulse patterns obtained with the single-nerve fiber action potential recording method and single-motor unit sEMG.
With the single-nerve fiber action potential recording it was shown that the neurons of the human CNS organize themselves by phase and frequency coordination. Following injury, this organization principle is disrupted and has to be repaired (see below). Another human electro-physiologic tool to measure natural impulse patterns of neurons is the surface electromyography (sEMG). With the same recording system used to record singe-nerve fiber APs, just replacing the wire electrodes with EMG surface electrodes, single-motor unit firing and motor programs can be recorded non-invasively.
In Figure 5, the different frequency patterns of oscillatory firing of motoneurons are shown. Original records were taken with the single-nerve fiber action potential recording method from motoneuron axons and surface electromyography (sEMG) from single-motor units. a1-Motoneurons innervate FF-type muscle fibers and fire rhythmically with impulse trains consisting of 1 action potential in the order of 10Hz. a2-Motoneurons innervate FR-type muscle fibers and fire rhythmically with impulse trains consisting of 2 to 5 action potentials in the range of 4 to 7 Hz. The amplitude of the extracellular action potential of the a2-motoneurons (axon group diameter = 10.2µm, axon group conduction velocity = 50m/s) is on average smaller than that of the a1-motoneurons (axon group diameter = 13.1µm, axon group conduction velocity = 65m/s), depending on the position of the axon in the nerve root with respect to the recording electrodes. FR-type motor unit potentials have much smaller amplitudes than the motor unit potentials of FF-type muscle fibers. The a3-motoneurons (axon group diameter = 8.3µm, axon group conduction velocity = 37m/s) innervate S-type muscle fibers and fire oscillatory at a frequency of around 1 Hz with long impulse trains (up to 50 action potentials per impulse train). The motor unit firing of single S-type muscle fiber motor units could not be safely identified by sEMG because their amplitudes are still smaller than those of FR-type motor units and are thus difficult to identify. The impulse patterns of oscillatory firing motoneurons obtained with sEMG are similar or the same as those obtained with the single-nerve fiber action potential recording method (Figure 5). This confirms the accuracy of the single-nerve fiber action potential recording method. Since sEMG is a non-invasive recording method, oscillatory firing can be recorded easily when using appropriate patients.
Integrative Physiology: System Theory of Pattern Formation
To understand the on-going changes of movement and other patterns in healthy humans and in patients with CNS injury, malformation and degeneration (aging), the System Theory of Pattern Formation is used for understanding neuronal network organization and learning.
In a complex system like the human CNS, patterns are generated by a nervous system which seeks cooperative stability. Stability is what defines collective states. The system has the tendency to slip into the collective states to which it is attracted. When an infant crawls, its arms and legs are strongly attracted to the ‘pace’ and ‘trot’ gait patterns. The attraction is so strong that intermediate crawling patterns seemingly do not exist, as if the patterns are hard-wired. But with the help of the special CDT device the CNS can generate intermediate coordination patterns. A patient with a CNS injury often crawls with intermediate arm and leg coordination patterns and has to re-learn the pace and trot gait coordination’s for CNS repair and shifts in this way the attractors for crawling to the pace and trot gait coordination’s. Attractive states and attractors of CNS organization can be pictured as a ball in a potential well or more generally in an attractor layout. Changes in CNS functioning are characterized as continuous stabilization and destabilization, over time, of preferred attractor states.
If for example a therapist is crawling in interpersonal coordination with a cerebral palsy girl, the learning process is speed up. The visual input from the exact crawling of the therapist into the CNS of the girl improves the performance of the crawling pattern. For this supervised learning the cerebral palsy girl needs not to concentrate to it. It is working automatically. This interpersonal coordination is something like when soldiers march together. Once they got the rhythm among each other, the marching coordination works automatically. It was even reported that soldiers could march together in interpersonal coordination when half sleeping.
This supervised teaching of the therapist, so that the patient learns faster needs a lot of concentration. The therapist has to copy the pattern of the patient and has to drag her then into a better performance. In doing so, the therapist sometimes is also losing the own movement pattern. With the concentration on the patient and adapting partly to the pattern of the patient, the stability of the own movement pattern is reducing strongly and easily lost.
To reduce for understanding the complexity of human neural networks of the many billions of neurons, order parameters or collective variables are introduced for the generation of certain movements. An equation of motion describes the coordination patterns dynamics. However, coordination patterns are not only determined by the task or biological function. Patterns adjust continuously to requirements from the environment (transmitted by impulse patterns from stimulated receptors in the periphery), memory, intention, and support given by a therapist. The specific requirements are captured by the concept of behavioral information and are made part of a vector field that attracts toward the required patterns. The coordination pattern dynamics, characterized by equations of motion of collective variables (the vector X), takes the general following form [39]:
(2)
Where Fintr designates the intrinsic dynamics of the nervous system. These intrinsic dynamics capture the anatomical (neural network structure), physiological and pathological states of the CNS and its muscular-skeletal elements.
∑cinfFinf(X,t) represents the sum of external influences (Finf(X,t)) with their relative strength (cinf) pertaining to each influence. The so-called behavioral information Finf(X,t) includes cognitive states, emotional states, intentions, motivations, instructions, inter-personal coordination, movement support etc. During motor learning or while applying therapy to a patient these extrinsic influences become extremely important, because the intrinsic (pattern) dynamics can be changed with these extrinsic influences by altering the equation of motion. By modulating the behavioral information, the intrinsic dynamics of the neuronal networks can be influenced further, that is if CDT is no longer efficient in repairing the injured CNS, the therapy has to be updated. With respect to a healthy athlete, the movement performance can be improved by modulating the behavioral information by for example including in the training program the exercising on a special CDT device to improve CNS functioning.
If the behavioral information includes the exercising of extremely coordinated, integrative movements, like exercising on the special CDT device, the quality of CNS self-organization can be enhanced by improving the exactness of self-organization, namely the precision of phase and frequency coordination between neuron and neural assembly firings. By improving the precision of organization of the intrinsic dynamics, that is the specific variability of the injured networks, certain patterns do eventually re-appear in the case of repairing the injured CNS by movement-based learning.
Learning implications for treatment derived from the equations of motion of the collective variables in the framework of System Theory of Pattern Formation
- Behavioral requirements Finf (like intention, support, and instruction) affect the whole coordination dynamics, including stability, rather than only certain coordination patterns. The change of the whole coordination pattern dynamics of the CNS by the behavioral information is one scientific basis for learning transfer between different patterns and stability changes of patterns (as for example the reduction of spasticity). The other scientific basis for learning transfer is followed from human neurophysiology, namely that nerve cells or neural sub-networks are involved in different neural network organizations [1].
- Intrinsic coordination tendencies captured by the intrinsic dynamics influence the performed pattern systematically because the degree to which intrinsic tendencies conflict or agree with the required patterns determines the variability of the performed coordination pattern.
- A reduction in stability of movements and other patterns when intrinsic and informational requirements conflict, may lead to loss of stability and abrupt change while behavioral information is changing smoothly.
- The intrinsic dynamics Fintr include vegetative and higher mental functions (these are also patterns of the coordination dynamics), which indicate that via exercising coordinated movements with support and/or instructions (Finf), urinary bladder function, intelligence and speech may be partly repaired or improved following CNS injury or malformation.
- When in an injured CNS with a certain set of behavioral information (∑cinfFinf) the intrinsic coordination dynamics (Fintr) can no longer be influenced during coordination dynamics therapy, then this set of behavioral information has to be changed (using different Finf), or balanced differently (using different cinf), to further improve CNS organization dynamics.
- However, the equations of motion of the coordination pattern dynamics (formula 2) provide no information about the specific behavioral information (Finf) and training intensity (cinf) with which the CNS can be efficiently repaired by learning in the patient. We need to have detailed knowledge of the human CNS at the single neuron and neural assembly level [1], as well as knowledge at the integrative level, to find the specific behavioral information for the repair by learning of the human CNS.
A first novel step in coordination dynamics therapy is the inference derived from the formula 2 of the equation of motion. It suggests that the movement learning not only improves the performance of that particular movement, but also improves other non-trainable functions by transfer of learning [40]. These functions include vegetative functions like urinary bladder control, speech (if the patient cannot speak) and higher mental functions.
Furthermore, we have a means by which the stability of physiological network states can be increased (e.g., movements, continence, continuous concentration in performing certain tasks, speech etc.) and simultaneously the stability of pathological network states, like spasticity, decreased. The coordination (pattern) dynamics therapy, partly based on the System Theory of Pattern Formation in combination with human neurophysiology (including neuro-urology), thus offers us an important theoretical basis and a practical tool to diagnose, quantify and repair/improve the functioning of the human nervous system at the macroscopic level. Through neural network learning we can reach for repair the whole CNS, including the sacral and pontine micturition centers and the plexuses outside the CNS.
From the repair by learning in the severely injured CNS we can learn about learning in the healthy CNS.
Geographical landscape of attractors
The drawback of the equation of motion of the order parameters (formula 2) is that it is normally not possible to find a mathematical solution to it. But by defining a potential function and by picturing the attractive states and attractors by a ball in a potential well or rather by a ball moving in a geographical landscape of attractors (Figure 6), we form a theoretical basis to understand and measure stability of certain coordinated movement patterns (i.e., the deepness of the potential well of an attractor) in patients with CNS injury who receive on-going therapy.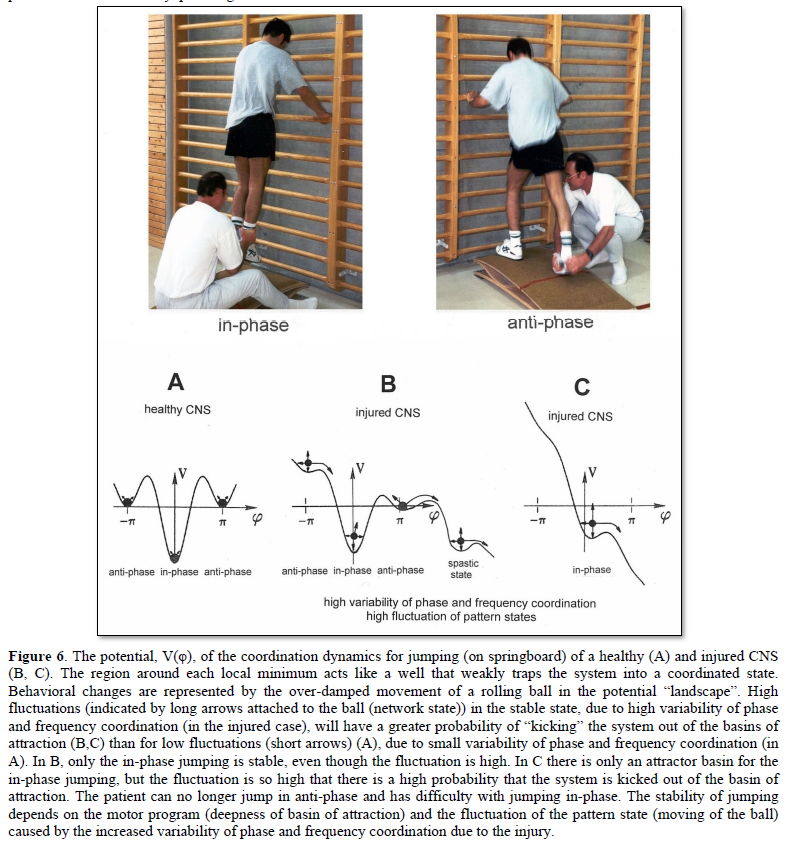
By studying the pattern change of certain highly coordinated arm and leg movements, while a subject is exercising on a special coordination dynamics therapy device, pattern stability can be made visible and the mean stability per one minute can be measured by the arrhythmicity of exercising. Such value, so-called coordination dynamics value, quantifies CNS functioning objectively, integrative, and non-invasively. The assessment of quality of CNS organization by pattern change is a second novel step in CDT.
To make the strategy of pattern formation, pattern stability, pattern assessment, and pattern picturing understandable, the procedure is demonstrated for the simple movement ‘jumping on springboard’, which is used during CDT, especially for the repair of the urinary bladder and training in the up-right weight-bearing posture
Equation of motion, potential function and attractor layout for the movement ‘jumping on springboard’
For the special movement ‘jumping on springboard’ with no behavioral information (∑cinfFinf(X,t) = 0) the equations of motion (formula 2) take the form:
Where φ is the relative phase between the two moving legs and is the only collective variable of this special movement.
The mathematical solution of dφ/dt = fintr(φ) in the Haken-Kelso-Bunz model [39,41] (for the approximations being made) gives the equation of motion for jumping on a springboard for the space-time symmetry:
The so-called potential function is defined by
By integration we obtain the potential function for jumping on a springboard:
For an easy understanding, the potential function can be developed approximately as follows. With the space-time symmetry V(φ+2π) = V(φ) (time symmetry) and V(φ) = V(-φ) (space symmetry) and using the first two terms of the Fourier series with sines and cosines we obtain V(φ,t) = – a(t)cosφ – b(t)cos2φ by regarding that only cosines are invariant when φ is replace by – φ. The minus signs allow to interpret the function, V, as a landscape with attractor states for positive values of a and b [39, page 55].
The potential function V(φ,t) = – a(t)cosφ – b(t)cos2φ can be plotted for different φ and certain ratios of the parameters a and b and is shown in Figure 6. The variability of the pattern state jumping, caused by phase and frequency variability, is made visible by arrows in Figure 6.
In the healthy CNS, the phase and frequency variability is small (short arrows) and the jumping in-phase and anti-phase is stable (Figure 6A). Following injury, the potential landscape is deformed and the fluctuation of the network states, generating jumping, is high (Figure 6B). The in-phase jumping is still stable in spite of the increased fluctuation, because the basin of attraction is deep. The jumping anti-phase became unstable because the basin of attraction is shallow and the increased fluctuation in the state has a greater probability of “kicking” the system out of the basin. A switch into a spastic state is also possible. In severe CNS injury or malformation, the patient cannot jump any more in anti-phase because of the missing of attractors for anti-phase jumping (Figure 6C). Support is needed for anti-phase jumping (Figure 6, upper right). The jumping in-phase is still possible but unstable (Figure 6, upper left).
Upon performing very exact coordinated movements, imposed by devices, the nervous system of the patient learns to reduce the variability of phase and frequency coordination and achieves in this way a small fluctuation of the network states again as shown in Figure 6A. The progress in treatment (learning) is that the in-phase jumping in Figure 6C and the anti-phase jumping in Figure 6B become stable (Figure 6A) again. Also, the potential landscape will change due to the reduction of the phase and frequency variability. The important consequence for treatment is that when exercising on special CDT devices and reducing in this way the variability of phase and frequency coordination, the patient can induce the formation of patterns again, without having trained them (learning transfer). Through improving the coordinated firing of neurons, a cerebral palsy child will become continent and my become able to speak or may develop social behaviors.
In conclusion, the impairment of phase and frequency coordination, measured at the neuron level in human, can be included in the coordination dynamics at the collective variable level. The decrease of the variability of phase and frequency coordination (one kind of coordination repair) is an essential part of CNS development and repair by movement-based learning (neural network repair).
METHOD
Movements to be trained
Movements have to be trained which improve the coordinated firing of neurons, the phase and frequency coordination, and which make it possible that other parts of the brain/CNS take function over through plasticity. The phase and frequency coordination can be improved when patients exercise on a special coordination dynamics therapy device (Figure 7). The nervous system learns from the device to function more physiologically, including the sacral and the pontine micturition centers. With the repair of the phase and frequency coordination, some patterns, including continence patterns, already re-appear.
In severe central nervous system injury or lost brain parts, other parts of the nervous system have to take function over by plasticity. Therefore, also other arm, leg and trunk movements have to be trained as the automatisms creeping, crawling, walking, running and jumping with or without support. For bladder repair the exercising on the special device and the jumping on springboard are most important (Figures 6 & 7). The jumping on springboard activates the pelvic floor muscles rhythmically, of which the external bladder and anal sphincters are a part. When exercising on a special device, the neural networks of the central nervous system are activated and repaired in the deep complexity of neural network organization. This is achieved because arm and leg movements change their coordination between the paces and trot gait patterns. Through this pattern change, according to the system theory of pattern formation, the quality of CNS functioning can be measured through pattern change by a single value (see below). The movement-based learning process is optimal by training 15 to 20 h per week.
Unique properties of special CDT devices
The special CDT device has four important properties.
First, the patient performs coordinated arm, leg and trunk movements when exercising on it. The training of integrative patterns takes care of that the pathologic organization cannot escape from repair by shifting to another part of the CNS and the whole CNS, including the injured parts, is reorganized so that other CNS parts can take function over through plasticity.
Second, neurons are coordination detectors. Because the mechanical coordination between arm handles and leg pedals is extremely exact, the generated time-coordinated afferent input endplate potentials onto a neuron in the neural networks (approximately 5ms long) overlap more. The excitation threshold of the neuron is reached earlier. In this way, the efficiency of organization is improved. In spinal cord injury, for example, the transmission over the injury site will increase. If the mechanical coordination between arm handles and leg pedals of the device is not extremely exact any more, then the turning is only muscle training and not a training through which the nervous system can learn from the device to function better. Maintenance of the device and supervision by an educated therapist is necessary.
Third, the coordination between arm and leg movements changes from pace to trot gait, imposed by the device. The intermediate coordination patterns between pace and trot gait are difficult to generate for the CNS neural networks. If the patients CNS learns to generate these intermediate patterns, imposed by the device, then the neural networks have learned to function better (more precise) in the deep complexity of CNS organization. The patient’s nervous system learns by turning from the device, to function more physiologic through improving especially the phase and frequency coordination among neuron firings. This phase and frequency coordination can be measured by single-motor unit surface electromyography non-invasively and by the single-nerve fiber action potential recording method invasively. In Figure 4 the coordinated firing between a motoneuron and spindle afferent fiber was recorded and measured. This spindle afferent fiber contributed to the drive of the motoneuron, because of the constant phase drive. In Figures 4B & C, the phase variation was 1.4ms (3-1.6ms).
Fourth, the assessment of quality of CNS organization by pattern change is a third novel step in coordination dynamics therapy.
Measuring CNS functioning by the arrhythmicity of exercising (coordination dynamics value)
The impaired phase and frequency coordination at the single neuron level, the assembly level and the macroscopic level can be measured macroscopically when the patient is exercising on a special coordination dynamics therapy device (Figure 8) on which arms and legs turn with a slightly different frequency (transmission 19 (arms) : 18 (legs)). The phase coordination between arms and legs is imposed by the device. The loss of phase and frequency coordination between arm and leg movements becomes visible and measurable by the arrhythmicity of turning. During a turning cycle, the coordination between arms and legs changes between pace (P) and trot gait (K). According to the difficulty of the coordination, the turning frequency increases and decreases. This frequency variation (df/dt; f = frequency) can be recorded, quantified and displayed on a computer screen (Figure 8A) and is called coordination dynamics value. CNS functioning is therefore measured though pattern change (continuous pattern change from trot gait to pace gait) according to the System Theory of Pattern Formation.
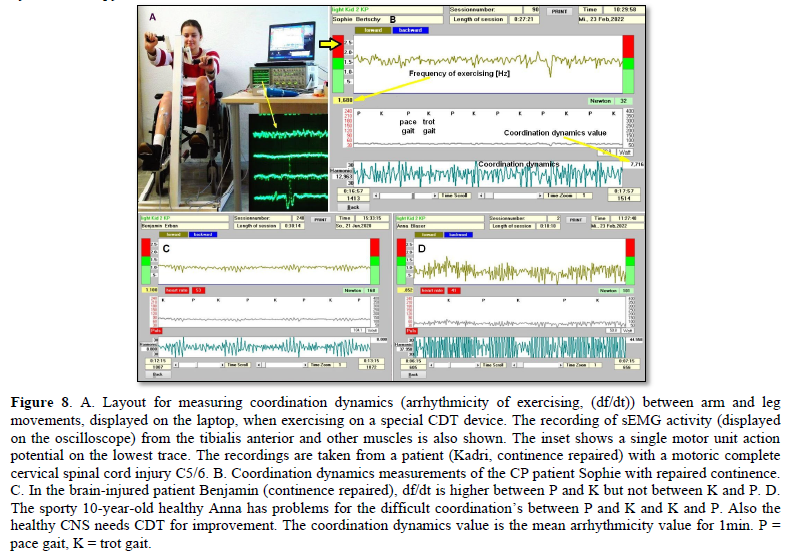
Brain-injured and healthy persons have problems with these difficult intermediate coordination’s between pace and trot gait, especially for higher loads (Newton) (Figures 8C & D), because the deep complexity of CNS organization is needed to generate these movement patterns. In reverse, if brain-injured patients learned to generate these difficult coordination’s, then their CNS improved in its functioning in the deep complexity of CNS organization.
During the functional reorganization of the injured CNS of patients, the relative phase and frequency coordination among neuron firings has to be entrained as exactly as possible by the movement induced afferent impulse patterns from the receptors (learning through feedback information) to restore coordination in the range between 3 to 5 milliseconds (approximate lengths of postsynaptic potentials). The device has therefore to impose the exercising patient a coordination in the millisecond range for the different coordination’s of arm and leg movements between pace gait and trot gait. The easy pace and trot gait coordination’s, but not the difficult intermediate coordination’s, can often be performed by the patient easily. Therefore, the continuous change from the easy to the difficult coordination’s diagnoses the capability of the CNS to organize easy and difficult organizational states. If the movement states can be easily generated by the neuronal networks of the CNS, then the frequency variation of turning is small during the turning cycle, and if the movement state is difficult to be organized by the CNS, then the frequency variation is large (the coordination dynamics value is large).
Repair strategies at the genetic level
For the repair of urinary bladder functions/patterns it is likely that excitation-neurogenesis coupling [42-44] contributed, stimulated through CDT. Repair depends on learning and memory formation, mediated or supported by epigenetic mechanisms. Epigenetics is the interplay between genes and the environment resulting in phenotype and epigenetic landscape. Epigenetic mechanisms, like DNA methylation, are probably sensors for movement-based learning and memory formation and fine modulators of neurogenesis though CDT. The hippocampus plays an essential role in learning and memory. In the hippocampus there exists a specialized form of neural plasticity, which is the generation of new functional neurons from stem cells occurring throughout life. Adult hippocampal neurogenesis contributes to learning and memory formation.
New neurons are important for learning and memory formation (besides functional reorganization), i.e., for increasing the rate of repair, for the following reasons:
- The insertion of new neurons helps to store the memory of the same activity that led to the creation of the neuron.
- Activity-dependent neurogenesis enhances the learning of new memories and degradation and clearance of previously stored unwanted memories like spasticity, because the synapses, dendrites and axons can be devoted more fully to the newer memories. The old neurons with large and complex axon and dendritic trees are difficult to change. They can only be changed with sustained effort.
- New neurons seem to improve the accuracy of relearned patterns (from model study [42]). This means that new neurons help to improve phase and frequency coordination of neuron firing and pattern stability.
- The advantage of new neurons seems to be dramatically greater in networks that had been more active and had been required to store more memories [42]. The advantage of neurogenesis for memory storage in heavily active networks is that it provides an increased rate of repair if movement-based learning is administered aggressively and if different movements are trained.
Specific natural network activity is required for multiple aspects of repair. Specific activity is essential for correct migration of interneurons and it also controls the development and repair of their axons and dendrites. During repair there is a specific requirement of network activity in shaping the cortical integration of specific neural subtypes. Newly build neurons are likely electrically active shortly after their birth and participate in the early network activity that contribute to circuit maturation during repair by CDT.
Specific activity is required for migration and maturation at several stages of repair. A break in CDT may invalidate the whole chain of repair events. Specific interneuron subtypes require activity for migration and morphological maturation at two distinct stages of development [42]. Newly built neurons may even require specific activity for migration and maturation at several distinct stages of repair. During a break in CDT, the specific activity, required for neuron migration, maturation and network integration may not be supplied at one of these stages so that the chain of repair events is severed and the whole repair chain has to be started anew. Drug application may undermine repair.
Excitation-neurogenesis coupling [42]:
- Excitation increases or decreases neuron production directly by excitation-neurogenesis coupling.
- The excitation acts indirectly on the surrounding mature (hippocampal) cells through depolarization-induced release of growth factors.
- Adult neurogenesis is enhanced by excitatory stimuli and involves Ca2+ channels and NMDA receptors.
Conclusion for optimal therapy according to the present stage of knowledge. If there is similarity between development and repair, animal (mice) data also hold in humans and the principles of neurogenesis of the hippocampus also hold in other parts of the brain, albeit to a much lesser extent, then the patient has to be trained at his limits (1) to induce substantial building of new nerve cells. The treatment has to be continuously administered (2) to support all stages of repair at the progenitor level as migration, maturation and integration. The networks requiring repair have to be activated specifically (3) to generate repair-friendly, micro-environmental properties in the networks. No drugs should be administered that change neuron excitability (4). The exercises have to include coordinated arm, leg and trunk movements (if possible) to improve the impaired phase and frequency coordination for CNS self-organization (5). The performed movements have to be as integrative as possible to reconnect distant brain parts and to induce learning transfer.
RESULTS
Summary
Urinary bladder function repair in a group of patients through Coordination dynamics therapy
In 7 spinal cord injury and 3 cerebral palsy patients, urinary bladder functions were improved. Incontinence was repaired in 5 spinal cord injury patients and all 3 cerebral palsy patients. The repair time depended on the severity of the brain or spinal cord injury and the intensity and duration of the treatment. In the cerebral palsy patients, the repair time was approximately 3 months. In a 50% cervical spinal cord injury, the repair time was approximately also 3 months. In a rather complete cervical spinal cord injury (95% injury), the repair time was 2.5 years. In a complete thoracic spinal cord injury (100% injury) the bladder was not repaired within 2.5 years. Additional stem cell therapy did not help. Obviously, in severe spinal cord injury, the bladder repair is very difficult, but important. The by movement-based learning stimulated nervous system has to use all available repair mechanisms in and outside the spinal cord, including genetics. Urinary bladder repair is the biggest problem in spinal cord injury. Ongoing urinary bladder infections ruin the bladder wall and stimulate bladder receptors to function pathologically, which is measured in detail by a comparison of the activity of stretch, tension and flow receptors in a paraplegic patient and a brain-dead human. Through coordination dynamics therapy the immune system is improved in its functioning through movement-induced building of additional K-cells and improvement of cardio-vascular performance. Movements are repaired simultaneously. With the improvement of cardio-vascular performance, pressure ulcers do not occur any more in spinal cord injury patients. Exercising on the special coordination dynamics therapy device induces diuresis, which may be of importance in some patients and can support drug-induced diuresis. In cerebral palsy, simultaneously the higher mental functions were improved through neural network learning.
Urinary bladder repair in spinal cord injury
The most important functions to be repaired in spinal cord injury (SCI) are firstly the bladder repair, followed by the sexual function. The walking is on place three followed by spasticity and scoliosis.
Spinal cord repair depends on the severance of the injury. In the patient with a 50% SCI (Sten) the bladder was fully repaired through 2 months of CDT. In the patient Kadri with 95% SCI 2.5 years were needed. In a 99% injury the bladder was not repaired within 2.5 years. It is obvious from Figure 9 that possible repair depends strongly on the severance of the injury.
Because in 50% SCI the bladder is somehow functioning, animal researcher cut only 50% of the cord in animals to avoid urinary bladder problems, even though it is the most important function to be repaired in SCI.
In an almost complete cervical SCI, the caudal spinal cord is disconnected from the cerebral cortex and brain stem. With respect to urinary bladder functioning, the sacral micturition center is disconnected from the pontine micturition center (Figure 1A). Following the spinal shock some reflexes, such as the stepping automatism and urinary bladder reflex (similar to those in infants), may re-appear, especially when stimulated. Pathologic reflexes or automatisms also appear as for example extensor spasticity or spastic bladder. With very limited regeneration upon CDT, motor and vegetative functions partly re-appear in a cephalo-caudal direction and movements become controlled first proximally and then distally; spasticity reduces. This cephalo-caudal and proximal to distal scheme only partly holds, because the injured CNS uses all existing repair and adaptation mechanisms through movement-based learning, including plexus connections outside the spinal cord. But with respect to the improvement of trunk stability and breathing the repair in cephalo-caudal direction seems to hold.
To understand bladder repair through neural network learning and regeneration, the repair will be analyzed in the two patients Kadri and Nefeli.
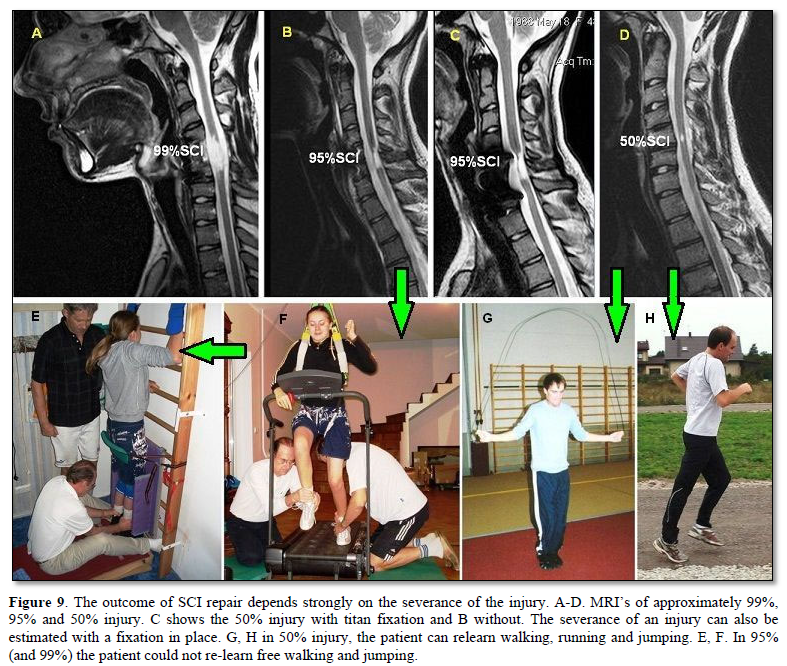
Urinary bladder repair in 95% spinal cord injury
The 17-year-old female patient Kadri suffered a severe cervical SCI in a car accident. No motor functions remained below the injury level of C5/6 and the patient had impaired feelings. From the MR images the author estimated that approximately 5% of the spinal cord matter was spared (Figures 9B & C). When the spinal shock faded away, it became obvious that no motor functions remained below the injury level but spot wise sensitivity remained more or less all over the lower body. Two months after the accident CDT was started. Upon 2.5 years of CDT the sensitivity improved and some motor functions returned below the injury level, indicating that some regeneration of the spinal cord had occurred. Urinary bladder functions were repaired. Details of the repair are given below (Figure 10). The connectivity over the injury site, according to the magnetic resonance imaging (MRI), may have increased to 8%.
Generally, a urinary bladder repair is very important in rather complete C5/6 SCI. The tetras are not able to perform intermittent bladder catheterization by themselves, because of the mainly lost finger functions due to the lost finger-function-motoneurons in C5/6 spinal cord segments. Through urinary bladder repair, the patients get their private sphere back.
Time course of the improvement of urinary bladder functions through CDT
It is reported about the stages of bladder repair through 2 years of therapy. The changes of functions of the detrusor (bladder) and the external and internal bladder sphincters are extracted from a detailed anamnesis and are pictured by an evolution of the attractor layout (in similarity to motor function of Figure 6) with the re-learning of bladder functions (Figure 10).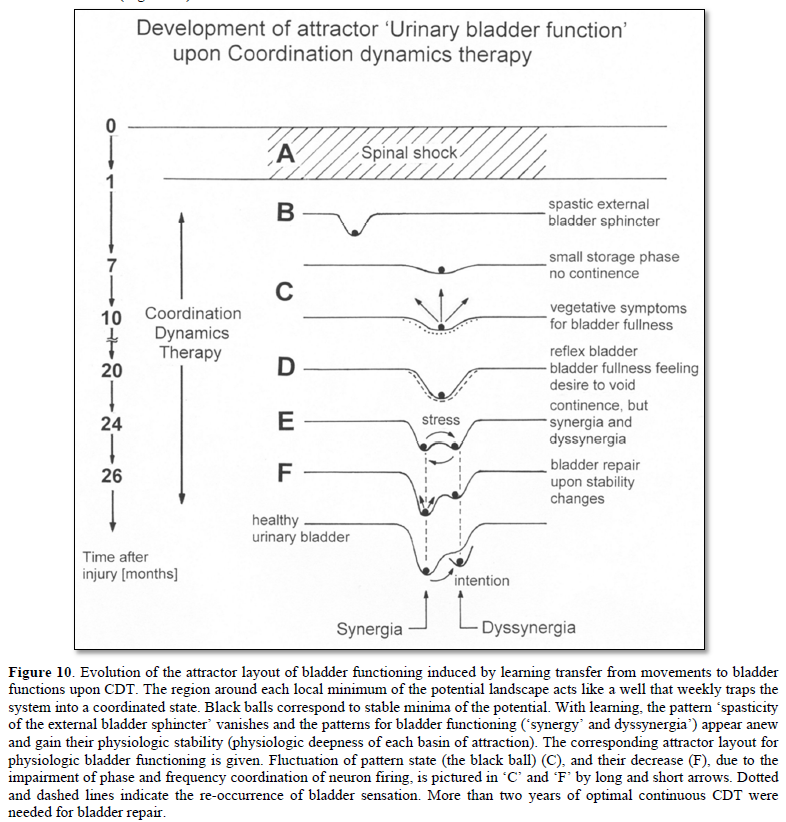
- After the operation (fixation and alignment of the broken cervical spine) a lying catheter was installed in the patient. The patient was suffering continuous infections and fever.
It is understood that the bacteria are ‘creeping up’ the lying catheter into the bladder (especially in female, because of the anatomy of the urethra) to give rise to ongoing infections in spite of antibiotic therapy. Before World War II (time of no antibiotics), patients were dying on such infections. It is the benefit of Sir Guttmann from Breslau [45] who stopped or reduced these infections by introducing sterile intermittent catheterization. The introduction of antibiotic therapy helped further.
- One month later (at a time when the spinal shock weaned) a suprapubic catheter was installed and no more infections occurred. But the bladder did not show any physiologic functions. The patient had no feeling of bladder fullness, no desire to void and did not feel when the fluid was leaving the bladder. The catheter was used for emptying when opened and storing when closed. Since no fluid was leaving the bladder through the sphincters, the external striated sphincter was spastic (continuous contraction) and the internal sphincter (smooth muscle), as a part of the detrusor, was probably not working.
Physiologically, the internal sphincter (smooth muscle, slowly acting, probably a part of the detrusor) is keeping the continence for low and medium bladder pressure. For high bladder pressure and sudden bladder pressure increases (as for example during coughing), the rather fast external sphincter (innervated by α2-motoneurons and consisting of fast fatigue resistant muscle fibers (FR)) is contracting to secure continence. If the striated external sphincter does not work properly, patients suffer the so called ‘stress incontinence’.
- Seven to eight months after the accident (end of 2005), the fluid was leaving the bladder by itself after a small storage phase. This means that therapy had reduced the spasticity of the external sphincter. The patient was now incontinent. So far, the spastic external sphincter had mainly stopped the fluid from leaving the bladder by its spasticity. The internal sphincter started to work a bit to allow a small storage phase. When the fluid was leaving the bladder, there was first no feeling of fluid movement. Later on, the patient had some feeling of fluid movement. Probably flow receptor afferents (S2) started to work. Three months later the suprapubic catheter was removed. The patient started to use diapers.
- 20 months after the accident (beginning of 2007, upon 18 months of therapy) the patient felt bladder fullness and the desire to void. Probably stretch (S1) and tension receptor connections (ST) started to work again. The vegetative symptoms of bladder fullness information (sweating and sudden heard rate increases, probably transmitted by plexus connections) were replaced by bladder fullness feeling and the desire to void.
- The patient became able to press the fluid out of the bladder. To get all fluid out, the reflex bladder had to be activated a bit, by tapping, touching or massaging the skin above the urinary bladder, which is the reflex skin area for the bladder reflex (Zones of Head). Sometimes body positioning was used to influence the bladder reflex. With these maneuvers the desire to void reappeared and the patient could empty the bladder further.
Often patients (to whom no CDT is administered) are training the bladder reflex for emptying. The reflex is stimulated by tapping the skin above the bladder. But if, for example, the external sphincter is spastic (as in this patient), it may not be possible to generate a good functioning reflex bladder. The neural networks of the sacral micturition center are working too pathologic.
- After the appearance of the desire to void, the patient became able to hold the fluid for 30s till 1min. Sometimes she could keep the continence better and sometimes not so good. This means that the external bladder sphincter (which can be controlled volitionally) started slowly to work, but irregularly. The feeling of bladder emptying became similar to those before the SCI.
- 24 months after the accident (spring 2007), the bladder started to function rather physiologically again. After a storage phase the fluid came out on volition. The detrusor started to work fully. But if the patient was pressing too much at the end of bladder emptying to get all fluid out (to reduce the residual urine, not to get bladder infections), the external sphincter contracted. The external sphincter co-contracted with the detrusor. Detrusor-sphincteric dyssynergia of the urinary bladder occurred (Figure 10E). But when she then activated the reflex bladder by tapping or touching the reflex bladder area, the desire to void reappeared and she could empty the bladder fully. The residual urine was not measured.
At this stage of bladder repair two patterns existed: the synergy of the bladder, in which the detrusor and external sphincter contracted antagonistically, and the dyssynergia of the bladder, in which the detrusor and the external sphincter co-contracted (Figure 10E). The synergy pattern was for emptying the bladder and the dyssynergia pattern was the pathologic pattern. The pathologic co-contraction of the external sphincter with the detrusor occurred more easily when there was less fluid in the bladder and the patient had to press more (inducing stress to the CNS).
Physiologically both bladder emptying patterns do exist. The synergy pattern is for emptying the bladder and the dyssynergia function is for stopping the micturition. But both patterns are under volitional control.
- Upon two years of CDT (26 months after the accident) the patient was full continent again and could empty the bladder on volition. The time interval from the first feeling of the desire to void to the situation that the fluid was coming out by itself (including 4 times of occurrence of the desire to void) was one hour. The patient did not use diapers any more. The patient had never used drugs which are supposed to improve urinary bladder functions. The repair of urinary bladder functions was achieved by the re-learning of urinary bladder functions including transfer of learning from the movements jumping on springboard, treadmill walking (Figures 9E & F) and exercising on the special CDT device. The strong improvement of urinary bladder functions occurred, when the coordination dynamics values strongly increased, indicating a bit of regeneration.
For patients with incomplete spinal cord injuries, it is very important how long they can hold the urine from the first desire to void till to the moment when the fluid comes spontaneously. Can they safely reach the WC or not? The improvement of bladder functioning can also be judged by how long the patient can hold the fluid following the first desire to void. In this case it was 1 h after 2 years of CDT.
The feeling of the lower abdomen, which was poor after the accident, improved also strongly at the time of nearly full bladder repair. The patient felt again the lower abdomen very good (inside and outside as the patient reported) and felt also again the working of the abdominal muscles. At that time, also the finger functions got a tiny bit better and her supported treadmill walking improved (Figure 9F). During walking on treadmill, and during other movements, the patient got goose-pimples all over the body. It seems that an overall improvement of vegetative and somatic functions occurred at the time of full bladder repair.
Attractor layout changes during urinary bladder repair
Within the framework of System Theory of Pattern Formation, the repair of the urinary bladder functions can be understood and pictured by the changes in the attractor layout.
One month after the injury, when the spinal shock faded away (Figure 10A, only the pathologic bladder pattern ‘spasticity of the external sphincter’ was present (Figure 10B). Six to 10 months later, the spasticity of the external sphincter reduced and a small storage phase of the bladder re-appeared as a first sign of bladder repair (Figure 10C). 20 months after the operation, the reflex bladder pattern organized itself with bladder fullness feeling and the desire to void (Figure 10D). 24 months after the accident, the attractor layout showed two attractors, the bladder synergy (the detrusor action inhibits the external sphincter) and the dyssynergia (co-contraction of detrusor and external sphincter) (Figure 10E). The state of the system (pictured by the ball) switched easily from the attractor synergy to the attractor dyssynergia. 26 months after the accident, the stability of the attractor synergy had increased and the stability of the attractor dyssynergia had decreased (Figure 10F). On volition (intention), the micturition could be induced and stopped as in healthy individuals.
Conclusion of urinary bladder repair in 95% spinal cord injury
Upon 2.5 years of CDT urinary bladder functions could be cured in severe (95%) cervical SCI. Since the injury was motoric complete and the cord was ‘free’ in the spinal canal (Figures 9B & C, the cord does not touch the vertebrae), some regeneration in the human spinal cord should have occurred.
Integrative functions and central pattern generators for urinary bladder repair
It was shown in this patient Kadri with a 95% SCI that urinary bladder functions could be cured upon 2.5 years of CDT. Three important steps were achieved. First, the patient got the bladder under volitional control again. Second, a physiologic attractor layout for bladder patterns could be generated; and third, the dyssynergia of the urinary bladder could be cured by increasing the stability of the synergy pattern and decreasing the stability of the dyssynergia pattern. The stability changes of the two bladder functioning patterns can be understood within the framework of system theory of pattern formation and human neurophysiology [1-3,46-48] but not in the framework of central pattern generators (CPG’s).
Repair of a 70% spinal cord injury at the level of Th10 (9-year-old girl Nefeli)
In the 5.5-years-old Nefeli a neuroblastoma was found to grow from the Th10 ganglion. With the surgery to remove the cancer she suffered a SCI at Th10/11 levels by medical malpractice. An 8-months-rehabilitation in Switzerland brought only little progress. Most of the repair was probably due to spontaneous recovery. When Nefeli started school, she was incontinent and could not walk. Assistance helped her to manage at school.
At an age of 9 Nefeli started CDT with the author. Spasmolytic drug and urinary bladder medications were stopped. Following four weeks of aggressive CDT, supported walking and jumping improved. The crawling became better. Following three years of CDT, Nefeli became mainly continent and she learned to walk with some balance problems. She learned to creep, crawl and jump. Even a bit of running became possible. At an age of 12.5 years, following 3.5 years of CDT, Nefeli learned to ride a normal two-wheel bicycle.
Repair in human patients with respect to animal research
The regeneration/repair in Nefeli [7,61] emphasizes the importance of continuous treatment on the long-term, especially in children during development. It would be interesting to see how animal researchers could simulate such repair over several years in rat or mice. In the patient Nefeli, the administered treatment lasted so far 3.5 years, in the former coma patient Manolis 7 years [21] and in brain injury patient Benjamin over 20 years. It needs to be known what repair can be achieved in human patients in what time period with an efficient therapy applied aggressively and continuously.
Causes of bladder dysfunction: bladder wall damage and neural network dysfunction of the micturition center
When the continence was repaired in spinal cord injury and cerebral palsy patients through coordination dynamics therapy (CDT), the question is “what was actually repaired when they became continent”. It will be indirectly shown that the bladder itself was repaired and the sacral and pontine micturition were repaired, that means the whole urinary system becomes repaired through CDT. It will be started with the bladder dysfunction in paraplegics. Its function is compared with the bladder function of the brain-dead human HT6, which is healthy in this respect.
Comparison of bladder afferent activity increase to retrograde bladder filling between a paraplegic and a brain-dead human
The pathology of the bladder afferent activity in the paraplegic 7 analyzed in Figure 11A, with dyssynergy of the bladder, is fully revealed by a direct comparison with the activity increase of the bladder afferents following retrograde bladder filling in brain-dead human HT6 (Figure 11B). The dependence of the bladder afferent activity on the bladder filling volume in paraplegic 7 shows four main differences in comparison to those in HT6. (1) The bladder afferents already fired with no bladder filling which was not the case in HT6. (2) The activity increased more smoothly in HT than in the paraplegic, even though bladder filling was stopped twice in HT6 but was not stopped in Para 7. (3) In the Para 7 the tension receptor afferent activity was higher than the stretch receptor afferent activity which was not the case in HT6. Especially the tension receptor afferent activity was several times higher in the paraplegic than that in the brain-dead human, the latter probably representing the physiologic case in this respect. (4) The bladder afferent activity was higher in the paraplegic than in the HT6. The finding of increased afferent activity in two paraplegics is in accordance with the experience that in brain-dead humans’ activity from bladder afferents was recorded only occasionally, whereas in paraplegics the activity from S1 and ST afferents was always obvious and prominent in the velocity distributions, rather independent of the extent the bladder was filled. Paraplegics with dyssynergy of the bladder suffer from high bladder pressure. This increased afferent activity will have consequences on the excitation of the CNS activating the striated sphincters of bladder and rectum.
Increased bladder afferent activity a reason for urinary bladder dysfunction
The dramatic increase in the activity of the stretch (S1) and tension receptor afferents (ST) was shown with certainty in two paraplegics (Figure 11). Also in several other patients’ bladder afferent activity seemed to be increased as the bladder afferent activity was prominent for even small bladder filling volumes. Nearly all patients who underwent surgery (for implanting an electrical bladder stimulator) had a much smaller storage volume of the urinary bladder, but not necessarily a reduced compliance (filling volume / pressure ratio). Following urinary bladder de-afferentiation the bladder storage volume increased as did the compliance. However, there are also paraplegics with a storage volume of 50 ml and no increased compliance. They are said to have hyper-reflexia of the bladder. Thus, there are patients with a stiff and infected bladder in whom the afferent activity of the bladder is much higher. The afferent activity is even often present with an empty bladder. The increased bladder afferent activity causes the detrusor being activated too early. On the other hand, there are patients with no increased compliance and no infection, and a detrusor which already contracts at filling volumes of 50 ml. It will be shown that there are important changes in the somatic and parasympathetic neuronal network of the sacral micturition center and in the coordination between them; this can account for an early activation of the detrusor and the external sphincter. Most of the complications of urinary bladder functions can be avoided and urinary bladder functions be cured if CDT is started early after spinal cord injury.
Premature activation of sphincter motoneurons in paraplegics following too high input from tension and stretch receptor afferents
Since with the single-nerve fiber action potential recording method the activity is recorded simultaneously from afferent and efferent fibers in thin sacral nerve roots, it is possible to analyze bladder functions with the inclusion of the activity of sphincteric and pelvic floor motoneurons. It can be measured at what filling volumes the sphincteric motoneurons start to fire in response to retrograde bladder filling in patients with SCI and in brain-dead humans. It can be measured with what afferent input to the sacral micturition center, induced by bladder filling, the motoneurons, securing continence, is activated. In consequence, also dysfunction of the sacral micturition center can be analyzed.
Figures 12A & B illustrates the activity increase of motoneurons, innervating the external bladder sphincter upon retrograde bladder filling and illustrates simultaneously the activity increase of motoneurons with tension receptor afferent activity changes in a brain-dead individual and in the paraplegic 7.
In the brain-dead human, the sphincteric motoneurons show only a little activity in the occasional firing mode for a bladder filling smaller than 500 ml (storage phase). The activity of the bladder tension receptor afferents is nearly zero for no bladder filling. In paraplegic 7, the sphincteric motoneurons a2(1) and a2(2) fired already oscillatory to generate high activity levels when the bladder was still empty. Since in the paraplegic the activity of the tension receptor afferents was already very high for the empty bladder, the high excitement of the sphincteric motoneurons was most likely caused by a high bladder afferent activity, especially from the tension receptors of the bladder wall.
With increasing bladder filling, the activity of the sphincteric motoneurons increased in a manner similar to that observed in the brain-dead individual, only at much smaller filling volumes. By comparing the activity increases of sphincteric motoneurons and tension receptor afferent fibers in paraplegic 7 with those in the brain-dead individual, it is concluded that the sphincteric motoneurons in the paraplegic behaved similar to the motoneuron in the brain-dead, the latter possibly representing the physiologic case in this respect. Only the sphincteric motoneurons in paraplegic 7 were activated too early because of a too high afferent input. The too high activity of the bladder afferents in the paraplegic mimics a rather full bladder, even though the bladder is still empty. The storage phase of the bladder is lost because of the too high afferent input. If the detrusor is also activated at certain bladder afferent inputs, similar to the sphincteric motoneurons, then the detrusor will also be activated at too small storage volumes. This means that the detrusor in this paraplegic was activated too early because of the too high bladder afferent activity at small bladder filling volumes. The so-called hyper-reflexia of the bladder, namely the automatic detrusor activation for too small bladder volumes in this case, can be explained by the too high bladder afferent activity, especially from the stretch receptors of the bladder wall.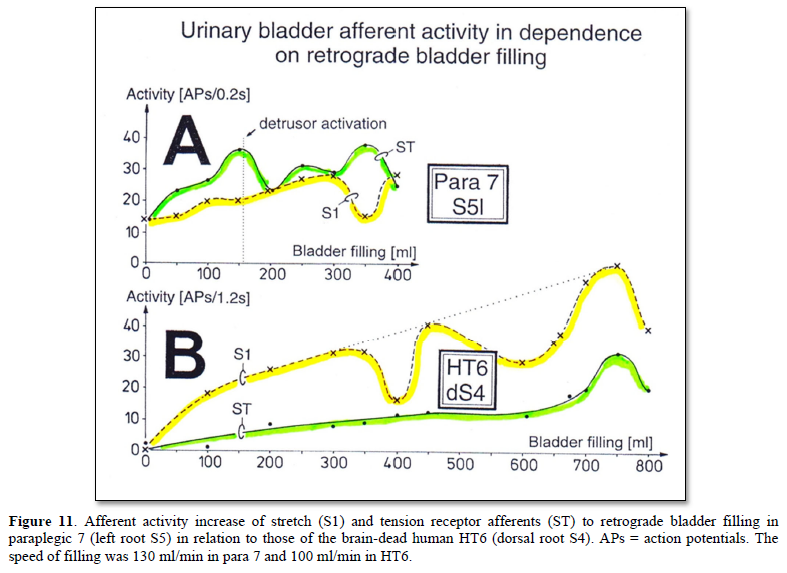
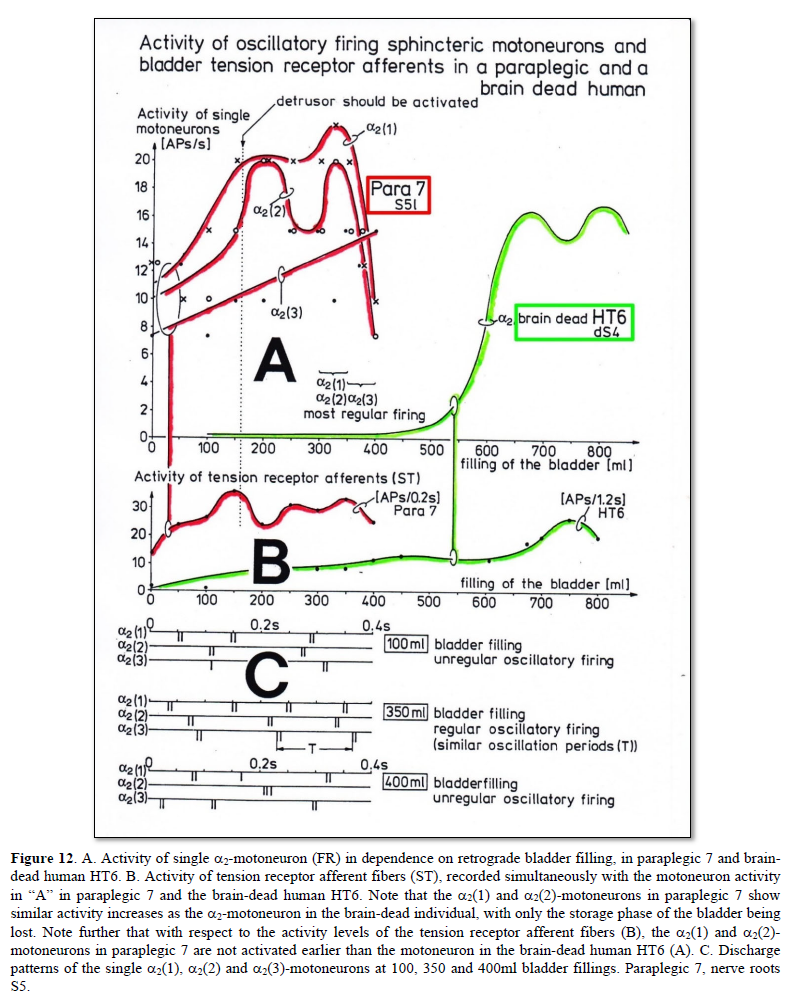
In the brain-dead human the sphincter a2-motoneuron fired in the occasional firing mode at low bladder filling, in the transient oscillatory firing mode at bladder fillings between 500 and 600 ml, and in the continuous oscillatory firing mode at high bladder filling (Figure 3). In the paraplegic, the a2(1) and a2(2)-motoneurons, innervating the external bladder sphincter, fired continuously oscillatory for any bladder filling.
The motoneurons innervating the external bladder sphincter are rather specifically driven by the bladder afferents. The secondary muscle spindle afferents (SP2), activating motoneurons that innervate the external anal sphincter, did not increase their activity with the increasing bladder filling and, for little bladder filling, did not contribute to the high activity of the a2(1) and a2(2)-motoneurons innervating the external bladder sphincter.
During surgery, the detrusor was not activated upon bladder filling (see discussion in [49]). Before the surgery, the detrusor was activated at approximately 160 ml bladder filling, at a volume when the sphincteric motoneurons a2(1) and a2(2) showed their first activity peak. If the parasympathetic division had not been suppressed by anesthesia, it is likely that the detrusor would have been activated at that filling volume during surgery. The motoneurons normally are activated strongly at high bladder filling to secure continence. At those bladder fillings, the urge to void is probably high. If in paraplegic 7 the bladder sensibility had been preserved, the urge to void would have been strong for small storage volumes. The relative extensive de-afferentation of the bladder in paraplegic 7 - cutting of dorsal roots S2 to S5 for implanting an electrical bladder stimulator (S5 root was sometimes not cut, if many parasympathetic fibers were contained), ventral root afferent fibers and possible bladder afferents running through the plexus hypogastricus remain preserved - increased the storage volume from 160 to 500 ml and the compliance from 19 to 38.
In Figure 12A, there is another a2-motoneuron (a2(3)) that increased its activity upon bladder filling. Since this a2-motoneuron (FR) showed a different activity increase than the a2(1) and a2(2)-motoneurons, it is likely that this motoneuron did not contribute to continence. It was activated by reflex or response generalization. In Figure 12C, the impulse patterns of the a2(1), a2(2) and a3(3)-motoneurons are shown for different bladder fillings. The motoneurons fired mainly rhythmically with impulse trains consisting of two action potentials (APs), in accordance with the expected pattern of bladder sphincter motoneurons [34]. In accordance with the measurements in the brain-dead individual (Figure 3), the oscillatory firing is most regular for the highest motoneuron activation and less regular for smaller bladder fillings and probably overfilled bladders. But the oscillatory firing neuronal network, driving the motoneurons, was dysfunctional, as the oscillation period was strongly changing. In paraplegic 9 the disorder in the neuronal network of the functionally disconnected spinal cord was so grave that even the oscillation period and the number of APs per impulse train changed.
In the next section, the interaction between the somatic and the parasympathetic neuronal networks will be considered at points where the detrusor activation is expected to inhibit the motoneurons innervating the striated (external) bladder sphincter. Again, recordings obtained from the brain-dead individual HT6 with bladder synergia will be compared with those from a paraplegic with detrusor-sphincter dyssynergia.
Coordination impairment between the somatic and parasympathetic nervous system divisions in the human sacral micturition center following spinal cord injury
After having shown that the identification of somatic and parasympathetic nerve fibers by the group conduction velocity and the group nerve fiber diameter is possible (Figure 13) and had not changed following spinal cord injury (SCI) [1], it will be analyzed here, on the basis of natural impulse patterns, travelling into and out of the sacral micturition center, what became pathologic in the organization of the CNS following spinal cord injury.
Normal voiding is a voluntary act which results in sustained contraction of the bladder (detrusor) and relaxation of the urethra until the bladder is empty. To enable fluid flow along the urethra it is necessary that the pressure in the urinary bladder exceeds that within the urethra lumen. Under normal circumstances, in order to initiate micturition, a fall in urethral pressure immediately precedes a rise in pressure within the lumen of the bladder. Usually, this pressure rise is produced by active contraction of detrusor smooth muscle at the onset of micturition. The extensive vesical part of the pelvic plexus and the profuse distribution of autonomic motor nerves emphasize the importance of the autonomic nervous system in initiating and sustaining bladder contraction during micturition. Immediately prior to the onset of micturition, the tonus of the striated external bladder sphincter is reduced by central inhibition of its somatic motor neurons located in the third, fourth and fifth sacral spinal segments. This inhibition is mediated by descending spinal pathways originating in higher centers of the CNS probably mainly the pontine micturition center. The central integration of the nervous control of the bladder and urethra is essential for normal micturition.
Immediately following complete SCI there is a period of spinal shock that lasts about three weeks or longer. This period is characterized by muscular flaccidity and a loss of spinal reflexes. The recovery of reflex activities below the level of the injury occurs at different times. If bladder reflexes reappear, they differ in some important respects from those in a normal individual. The ‘autonomic bladder’ contracts in response to distension, but the power is rarely that of the normal bladder, and the residual volume is increased. Injuries above the level of the brain stem centers lead to involuntary voiding in which the detrusor contraction is coordinated with sphincter relaxation (detrusor-sphincter synergy). Injuries below the level of the brain stem centers, but above the lumbosacral spinal cord, lead, after a period of bladder paralysis associated with spinal shock, to involuntary reflex voiding in which the detrusor (bladder) contraction is not coordinated with sphincter relaxation (detrusor-sphincter dyssynergy). The coordination between the detrusor and the external bladder sphincter will now be compared between a paraplegic patient with an injury below the brain stem and detrusor-sphincter dyssynergy (pathologic) and a brain-dead human with partly destroyed brain stem and an at least partial detrusor-sphincter synergia (partial physiologic).
If the SCI is complete, it is believed that paraplegics develop vesico-sphincteric dyssynergy because of the disconnection from the pontine micturition center which is responsible for the coordination of the detrusor and the external bladder sphincter. In cases in which no detrusor-sphincter dyssynergy has developed, the spinal cord injury is believed to be incomplete, so that supraspinal centers can still coordinate bladder functions. The external bladder sphincter will not be ‘co-activated’ with the detrusor in response to distension. The view that the important function of coordination is performed by spino-bulbo-spinal pathways has incorporated anatomical ideas of a hierarchy of functions of increasing complexity, the further rostral one goes in the CNS. Still, the coordination for the mutual inhibition of detrusor and external bladder sphincter may be located in the sacral micturition center. This dys-coordination problem shall now be analyzed using the single-nerve fiber action potential (AP) recording method. As was shown in the patient Kadri with a SCI (Figure 10), such a dys-coordination can be repaired by movement-based learning. The human nervous system has sufficient complexity for re-learning. Rats and cats may not have a sufficient network complexity for such repair [50-52].
Learning transfer is successful in repairing urinary bladder function, since the somatic and the autonomic divisions dovetail with one another with respect to jumping on springboard and exercising on the special CDT device. The necessary connection to the pontine micturition center for the coordination control of the detrusor and the external bladder sphincter is achieved upon CDT by limited regeneration of the human spinal cord, recruiting ectopic pathways through root connections, sympathetic chain or plexus connections (misdirected haphazardly during development) and re-organization of plexus outside the spinal cord. It is believed that the autonomic division of the nervous system is an elaborately organized division of the CNS with representations at all levels. At each level the somatic and the autonomic systems dovetail with one another.
For animals, reviews by de Groat [53] provide a basis for understanding how the filling and voiding functions work. He states that the primary stimulus for micturition is bladder distension, which induces reflex activation of the parasympathetic excitatory outflow to the bladder, depression of the sympathetic inhibitory outflow, and depression of the somatic efferent output to the external sphincter; secondary reflexes elicited by the passage of urine through the urethra may reinforce these primary reflexes and facilitate the complex emptying of the bladder. When the micturition reflex is exceeded, the parasympathetic reflex pathway through the brain stem is active. For an introduction to the physiology of the lower urinary tract, see [54].
It was shown that the activity in parasympathetic efferents can be measured, identified and distinguished from the activity of γ-motoneurons in conduction velocity distribution histograms (Figure 13). Since the action potential amplitudes of parasympathetic efferents is small, it would still be very difficult to analyze the organization of the parasympathetic nervous system and it’s coordinated functioning with the somatic nervous system as in the control of the urinary bladder. But if some secondary muscle spindles in the parasympathetic range are also innervated by parasympathetic efferents besides somatic efferents (γ-motoneurons), then the activation of the parasympathetic nervous system can also be measured by the activity of secondary muscle spindle afferents. Since the action potentials of secondary spindle afferents are comparably large (thick fibers), the activation of the parasympathetic nervous system can be easily indirectly assessed. It was shown that at least some secondary muscle spindles, in the parasympathetic range, are innervated by parasympathetic efferents (parasympathetic fusimotors) [55-58]. The evidence was obtained by measuring the parasympathetic activation of the detrusor by detrusor-pressure changes and by measuring activity changes of secondary muscle spindle afferent fibers and compare the form of the changes of detrusor pressure with the activity changes of a secondary muscle spindle afferent fiber [1].
Since in HT6, the somatic fusimotors did not change their activity levels strongly with the activation of preganglionic parasympathetic fibers and the activity increase of the secondary muscle spindle afferent fiber SP2(2) followed the transient increase of parasympathetic activity, it is likely that some muscle spindles are directly controlled by parasympathetic fusimotors. The direct control of some muscle spindles by parasympathetic fusimotors is supported by the slow activity decrease following very high activation [49] and the similarity with the time course of the detrusor pressure decrease following electrical stimulation of the preganglionic parasympathetic fibers during the surgery. The slow spindle afferent activity decrease would be difficult to explain by somatic fusimotor activation only. For further details see Chapter V of [1].
Detrusor-sphincter synergy of the bladder in the brain-dead human HT6, and dyssynergy in paraplegic 9
The detrusor-sphincter dyssynergy is analyzed now by comparing the natural impulse patterns of single secondary muscle spindle afferents (SP2) and sphincter motoneurons in a brain-dead human (physiologic) with those in patients with spinal cord injury (pathologic). The parasympathetic nervous system was activated by painful bladder catheter pulling (Figure 14).
Figure 14A shows that in the brain-dead human HT6, whose parasympathetic preganglionic neurons increased activity (Figure 14Ab) upon bladder catheter pulling, the SP2(2) fiber activity increased strongly (Figure 14Aa), whereas the a2-motoneuron, innervating the external anal sphincter, discontinued its oscillatory firing (Figure 14Ac), which is a measure for a strong activity decrease. A a2-motoneuron, innervating the external (striated) bladder sphincter, was not activated. This means that with the activation of the detrusor, the sphincter motoneurons were relaxed by inhibition. Thus, the brain-dead human HT6 had a detrusor-sphincter synergy of the bladder.
In the paraplegic 9, the SP2(1) fiber showed no strong activity increase and there was no sphincter relaxation following bladder catheter pulling (Figure 14B). The secondary muscle spindle afferent fiber SP2(1) increased its activity in an undulating manner (Figure 14Ba). The parasympathetic fusimotors, driving the muscle spindle, innervated by the SP2(1) fiber, probably were not continuously active as suggested by Figure 14Bb, in contrast to the parasympathetic activity observed in the HT6 (Figure 14Ab). The other secondary muscle spindle afferent fiber in paraplegic 9 (SP2(2), Figure 14Ba) slowly reduced its activity upon bladder catheter pulling. This spindle afferent fiber was not connected to the continence of the bladder. It is likely that its spindle was not parasympathetically innervated and was sited in leg muscles or parts of the pelvic floor muscles not contributing to continence. The a2 and a3-motoneurons (Figure 14Bc) showed a high activation, which is expressed in their oscillatory firing, and probably contributed to the continence of the bladder and the rectum. These likely sphincter motoneurons did not reduce their activity following parasympathetic activation, as can be seen from the SP2(1) fiber activity, monitoring parasympathetic activity. These motoneurons were not inhibited and the external sphincters were probably not relaxed. The measurements in paraplegic 9 indicate a loss of the inhibitory action of the detrusor onto the sphincter motoneurons.
The time constant for the activity decrease of a spindle afferent fiber following parasympathetic activation was 31s in a paraplegic and approx. 40s in a brain-dead human (Chapter V of [1]). It is concluded that the muscle spindles are unchanged following spinal cord injury. The pathologic firing patterns of the SP2 fibers are thus probably a result of neuronal network changes in the parasympathetic and somatic nervous system divisions of the sacral micturition center.
In conclusion, in the brain-dead human the sphincter motoneurons sub-serving continence were inhibited at a time, when preganglionic parasympathetic efferents increased their activity (physiologic) for 10s and an SP2 fiber increased its activity for several minutes. In the paraplegic with a strong bladder dysfunction, the SP2 fiber activity increased, due to parasympathetic activation, lasted for approx. one minute, showed undulations, and its amplitude was smaller than that measured in the brain-dead human. There was therefore no powerful continuous parasympathetic activation and the sphincter motoneurons were not inhibited (pathologic).
Conclusion for the repair of the human CNS
In conclusion, following SCI the neural networks of the sacral and pontine micturition centers and their connections are functionally damaged and have to be repaired in similarity to the repair of motor functions where the intrinsic apparatus of the spinal cord is damaged. Spasticity has to be reduced and movement performance improved by improving the functions of the intumescentia lumbo-sacralis and supra-spinal centers and their connections. Neural repair means, the networks have to be repaired.
The research performed above, to clarify human urinary bladder functions under physiologic and pathologic conditions, is the level to be performed the Author expects from human brain research, where plenty of money is going to in USA and EU. Human research at the neuron level is also needed. With the clinical diagnostics as for example electroencephalography (EEG), magnetic resonance imaging (MRI) and positron emissions tomography (PET) one will never find out how the human brain is really functioning. For sure, human electrophysiology is needed for finding out how the human brain is functioning. It is impossible to understand why the discipline electrophysiology is world-wide not organized and used any more. The neural networks of the human brain are mainly functioning on the basis of voltages and currents and for measuring those electrical recordings are needed.
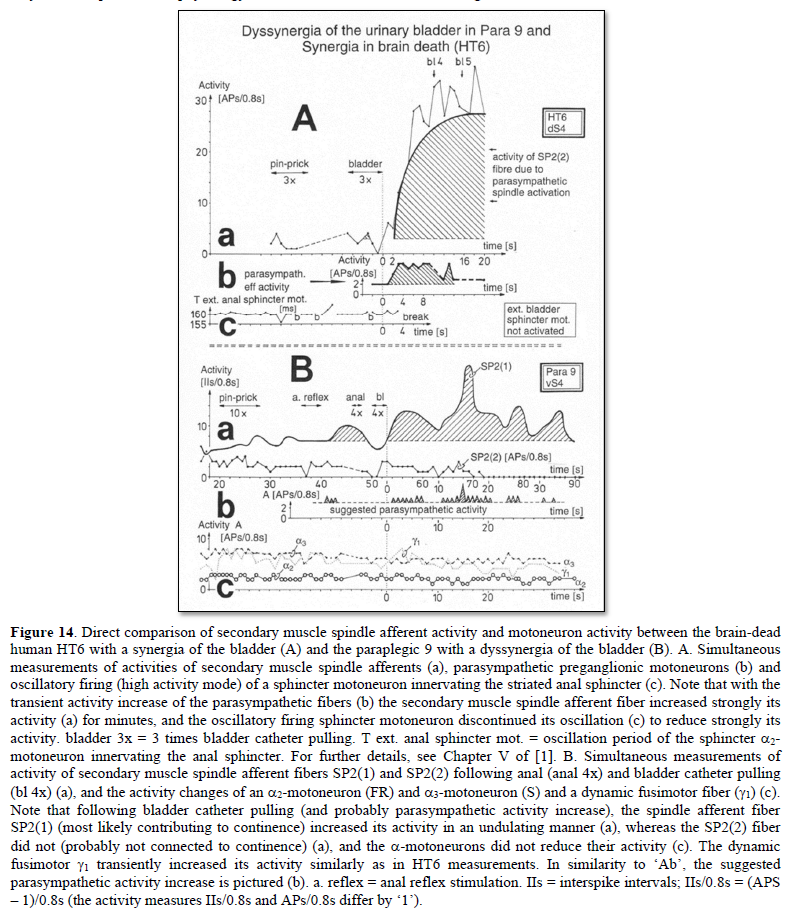
Continence repair through neural network learning induced by coordination dynamics therapy
Above it has been measured that bladder dysfunction occurs because of bladder wall damage, giving rise to pathologic high bladder receptor activity, and dysfunction of the sacral micturition center in connection with the pontine micturition center.
It will now be analyzed how coordination dynamics therapy can repair/improve bladder wall functioning and the neural network functioning of the sacral and pontine micturition centers. It is started with the neural network repair of micturition centers.
Continence repair mechanisms: Neural network repair through neural network learning of the sacral micturition center and bladder wall repair through healing all induced by coordination dynamics therapy
Following spinal cord injury, the oscillatory firing networks lose specific properties, which have to be repaired. The Eigenfrequencies of the premotor spinal oscillators change from a narrow to a broad frequency band (Figure 15).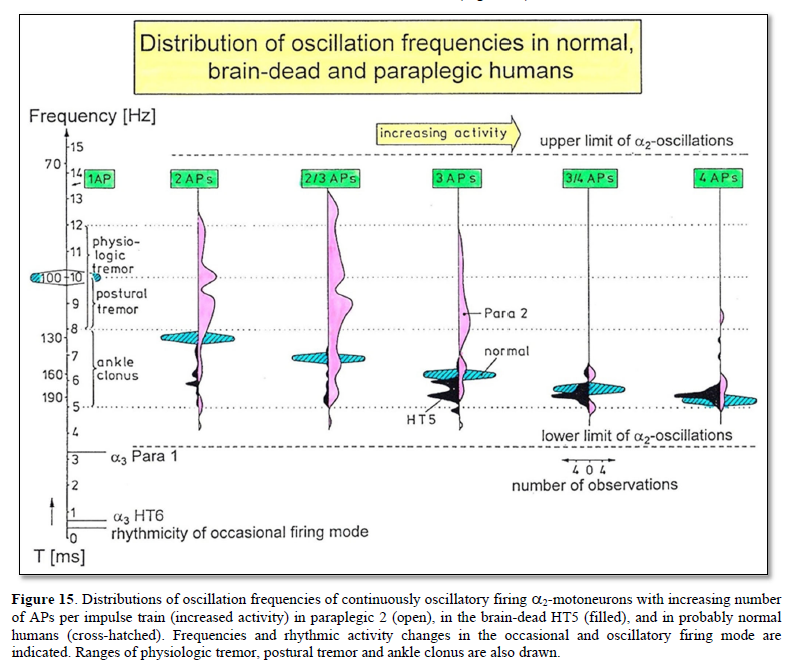
Self-organized α2-oscillators fire physiologically at an Eigenfrequency (varying probably within a small frequency band as indicated with the hatched distributions in Figure 15) with impulse trains consisting of two to three action potentials. Following brain death, this Eigenfrequency band enlarges (black area in Figure 15). Following a spinal cord injury, the Eigenfrequency band enlarges strongly and includes in this case the frequencies between 4 and 14Hz for firing with two or three action potentials per impulse train (Figure 15). The premotor spinal oscillators have lost their specific properties and could now be excited at frequencies at which they physiologically would not be excited. This is one reason for spasticity.
To repair the premotor spinal oscillators, it is used that they build up an external loop to the periphery through which they can be entrained for better functioning. An efficient way of entrainment is jumping on springboard.
Building up of external loops to the periphery by premotor spinal oscillators through which they can be entrained
A neural ensemble is built by efficiencies of synapses and projections between the convergence of several fusimotors (g-motoneurons) on one muscle spindle and by the divergence of muscle spindle projections onto several rhythmically firing populations of neurons driving a and g-motoneurons. Such a functional unit is partly pictured in Figure 16g.
With the building up of simultaneous phase relations between a, g and SP2 fibers, an external loop of premotor spinal oscillators is built up to the periphery, which makes it possible to directly influence the firing of spinal oscillators by a rhythm training (Figure 16).
Upon jumping on springboard (Figure 6) (and other rhythmic movements like sky-walking or running), premotor spinal oscillators organize themselves to fire transiently oscillatory according to the motor pattern and build up an external loop to the periphery (Figure 16). If the frequency of the rhythmic movement has an integer relationship to the Eigenfrequencies of the premotor networks and more rostral networks, these premotor networks get entrained for more specific self-organization.
If one assumes that loop circuits do not only exist between the premotor spinal oscillators and the periphery, but are a general structure in the CNS, then motor learning involves the formation of loop circuits (or better loop network circuits) between the cortex and the periphery involving the sensory cortex and the thalamus. When a linear oscillatory system is driven by an external periodic input its response contains both frequency components (the own and the external one). This is also, in general, true with nonlinear oscillators. However, in this case, if the external frequency is close to the Eigenfrequency of the oscillator itself, then it is possible to have a response at the external frequency only. This phenomenon is known as entrainment or synchronization. It is of paramount importance with respect to biological oscillators because it allows them to “latch on” to the environment. Thus, a rhythm with a free-running period of 24.7 h may be synchronized to 24 h when exposed to the natural sequence of day and night.
Upon jumping rhythmically on springboard (Figure 6), in addition to the stimulation of mechanoreceptors for movement control, also mechanoreceptors for bladder and rectum control are synchronously activated with the movement. Continence functions are synchronously activated with the jumping (coherent activation of bladder and movement patterns).
Repair of the impaired phase and frequency coordination through training very coordinated arm and leg movements on the special CDT device to allow specific patterns formation and learning transfer
The jumping on springboard is not sufficient for bladder repair, because the coordinated firing of neurons giving rise to CNS organization becomes impaired with every injury, malformation or degeneration. Therefore, self-organization of CNS networks by phase and frequency coordination has to be improved to make learning transfer from movements to bladder functions possible. Large instabilities in phase and frequency coordination will not allow specific pattern formation as a basis for learning transfer. However, the stability of phase and frequency coordination can be improved when the patient is exercising on special coordination dynamics therapy devices (Figure 7).
This training of very coordinated arm, leg, and trunk movements, imposed by the device, is especially improving the impaired phase and frequency coordination of neuron firing. The impaired phase and frequency coordination in CNS injury, malformation or degeneration turns out when comparing the coordinated firing of neurons in a brain-dead human (rather physiologic) with that of a patient with a spinal cord injury (Figure 17). Following injury, the network organization becomes deteriorated and escapes entrainment, learning, and learning transfer. But when patients exercise very coordinated arm, leg, and trunk movements on the special coordination dynamics therapy device for turning (on which a precise coordination between arm and leg movements is imposed by the device, to which the training individual has to adapt to), the CNS of patients can re-learn specific self-organization, which means can re-learn more exact phase and frequency coordination among neuron firings from the device. Since the CNS network is an open system, many different very coordinated integrative movements should be trained, not to allow the pathologic organization to escape from the repair/entrainment.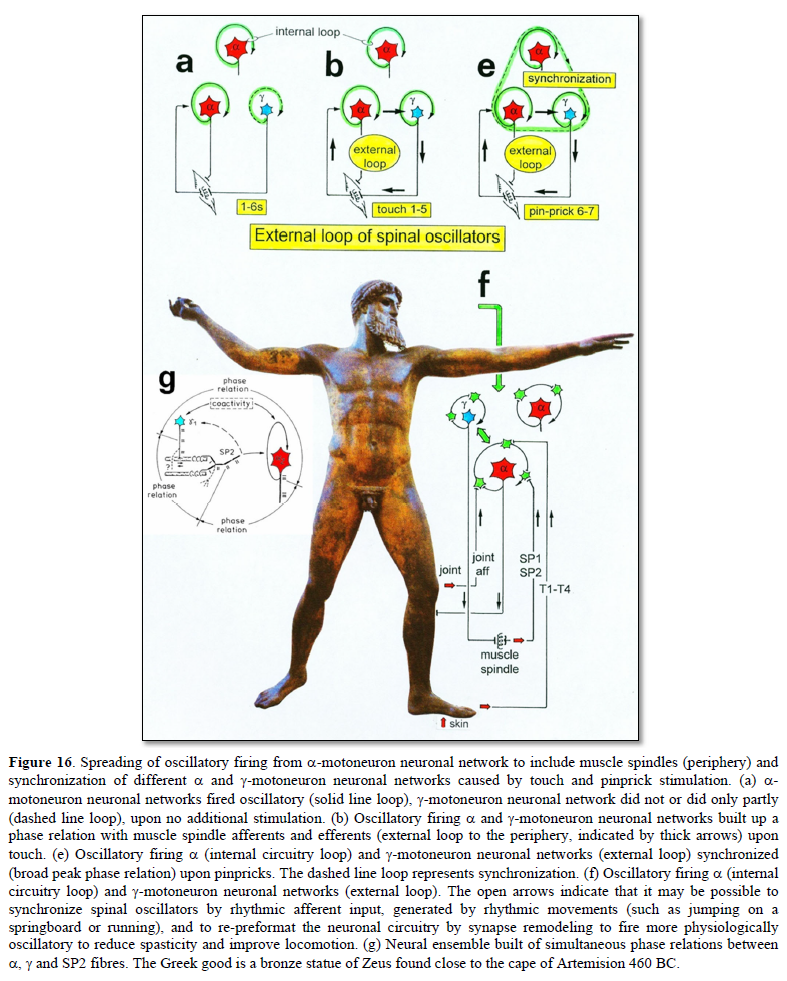
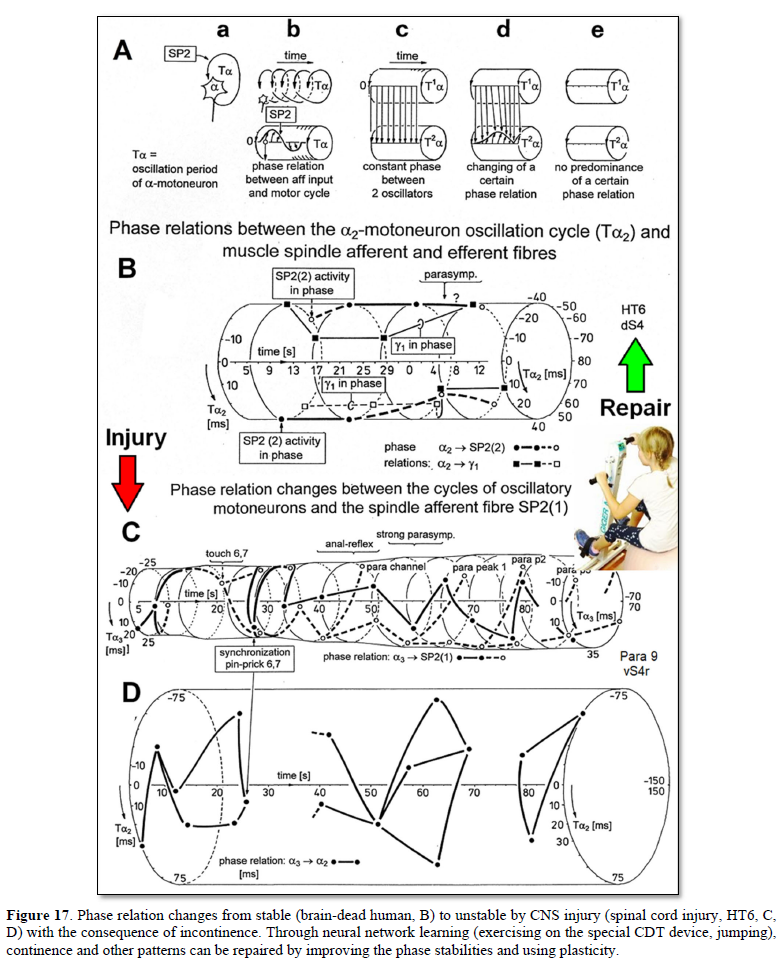
The more frequent occurrences of changes of phase relations between the different nerve fibers in combination with the changing number of phase relations per oscillation in the patient with a spinal cord injury (Figure 17) may mean that sub networks reacted and interacted more quickly and easily with others according to the afferent input. Especially because the oscillatory firing networks lost specific properties, their resonance frequencies changed from a narrow to a broad oscillator frequency band (Figure 15), which means that the oscillators were not excited at a certain frequency anymore but by a broad frequency band. They could now be excited at frequencies at which they physiologically would not be excited. Over-activation and mass effects could be the result. On the other hand, certain networks could escape from driving afferent influence by their phase change: phase escape to avoid interaction. Functionally far away networks are not reached anymore, which also would result in a loss of specific properties. Therefore, because of the loss of specific properties, some interactions could have occurred more easily and other ones not at all.
W.R. Hess tried, in 1944, to compare biological order and human society [59]. In a society, the upper behavior of spinal oscillators could be called “putting its flag to the wind.” There could be similarities between the organizations of the human nervous system and the organizations between very many individual nervous systems.
Bladder wall repair
Since coordination dynamics therapy (CDT) can improve the functioning of every nervous system, the healthy [60] and the injured one [61], and the nervous system is involved in nearly all body functions, the general health will improve when performing CDT. Via learning transfer, the functioning of the vegetative nervous system can be reached for repair/improvement from movement performance improvement. The bladder, including the bladder wall, can be repaired, even though it may take a long therapy time, depending on the damage infections may have done.
CDT speeds up the healing process by 20 to 30% and improves the healing result. When in the Author the maxilla was reconstructed by a tibialis transplant following cancer removal, the surgeon was amazed how quickly and good everything healed [22]. When the Author had a hip transplantation, the hospital stuff became nervous because he brought his special device into the hospital. But they were astonished that he was the first one who could leave the hospital. When spinal cord injury patients, performing CDT with the Author, got the spinal fixation removed, the healing process was faster than without CDT.
The question is, how can CDT improve the healing process and why never a patient got worse with CDT? The answer is very important with respect to live longer with a better quality of life [25].
It may be that CDT improves the coordination of the regulation processes in general (including the epigenetics) and improves in this way the healing processes, including the one for the bladder wall. Improvement of the cardio-vascular performance and building of NK cells [24] will contribute. Jogging and fast walking are also healthy and improve cardio-vascular performance and induce the building of NK immune cells. But first jogging does not improve the coordinated firing of neurons in the deep complexity of CNS organization and not many patients are able to jog ones a day for two hours or more.
Conclusion: Urinary bladder functions can be repaired and Continence research on human must be organized
Human repair-neurophysiology is a new discipline with which the human nervous system can be repaired. Since the nervous system is involved in nearly all body functions, the general health can be improved. Urinary bladder functions of the sacral micturition center can be measured and analyzed in detail by using the newly developed single-nerve fiber action potential recording method. By combining human neurophysiology with the System Theory of Pattern Formation, there is a theoretical basis that through movement-based learning vegetative and cognitive functions can also be repaired through learning transfer [62]. Bladder continence is achieved firstly via the repair of the vegetative (inner bladder sphincter) and the somatic nervous system divisions (external bladder sphincter) and their coordination and secondly via the improved healing process of the bladder wall induced by coordination dynamics therapy [65].
Proper basic human repair-neurophysiology [1-3,62] must start with the anatomy. The nerve fiber compositions of for example pelvic and vagus nerves and tract fibers in the spinal cord must be measured like in Figure 1. Action potential patterns of sets of single-nerve fibers must be measured simultaneously which are conducted in certain identified nerve fibers in and out of the sacral micturition center and CNS functions analyzed. Can a urologist, after working hard with the patients the whole day round, start with morphometry and electrophysiology in the evening even if he would have the knowledge, funding, and infrastructure? Human repair-neurophysiology is needed and must be organized. Statistics are important but not sufficient for the repair of the human CNS. In many cases incontinence can be cured through coordination dynamics therapy [65].
Yates [63] has 42 years ago constructively criticized the quality of research in the area of human urinary bladder physiology. In particular, he has recommended that investigators should look at the methods being used in parallel disciplines. Through basic and clinical research, urinary bladder functioning and repair has now been clarified and published [1,2,65]. At the continence conference 2022 of the international continence society (ICS), the e-poster of the Author (Figure 18) did not get questions touching the theory of bladder functioning and repair.

Epilogue
Ethics
After an initial financial support of this research project to repair the human CNS by the “Deutsche Forschungsgemeinschaft” (DFG), further support was refused because of professional and ethical doubts. Despite the support by the former German president Richard von Weizsäcker, the DFG did not change its mind and stated that the Author cannot apply any more for funding of this research project. Also, the “Swiss National fond” and the “Max Planck Institution” refused support. For the impossibility to get support for a research project to repair the human nervous system see Epilogue of [1]. Because of the impossibility to get official funding for this research project, the Author went on with the research, mainly on personally saved money for 37 years to reach the present level.
When the Author wanted to work on brain-dead humans at the University of Greifswald (at that time German Democratic Republic, or Russian occupational zone), mainly at the neurosurgery department [29], an ethical committee was founded and this committee decided that it is justified to work on brain-dead humans for repairing the human CNS. For developing the “single-nerve fiber action potential recording method”, the Author was supposed to get a professorship in Dresden (after being re-built). But after 10 years, with the fall of the Berlin wall, everything changed the bad and the good things.
The ethics in research are important. But it is not only important what research on human is justified, but also what research is allowed to leave out. Here an important example. Through coordination dynamics therapy it is possible to live longer with a better quality of life for 10 to 20 years [25,26]. If every hundreds person (10-2) on earth (world population = 7x1012) would have the mental discipline to train hard and live longer for 10 years (10-1), then every year a few million lives could be saved when assuming a life time of 100 years (7×1012×10-2×10-1= 7 million). That means qualified research on human has tremendous consequences.
War children (Kriegskinder)
Nightmares can partly be cured during CDT treatment of scoliosis and in cerebral palsy [9]. (The following is not easy to write for the Author, but he feels responsible to partly analyze the past).
However, the CNS of children cannot only suffer damage from a traumatic brain injury, infection, or malformation, but also from the fallout of conflict zones, especially in the absence of parents. They can suffer nightmares which may last for the rest of their lives. Figure 19 shows Butscha in Ukraine and Figure 20 shows the ruins of Dresden following the bombing in 1945. Such situation may induce nightmares in those ones who survived.


The Author walked through Berlin like the child in Figure 21. The streets were cleaned by women (Trümmerfrauen). The bricks in the ruins seem to have been turned several times by the bombing.
To imagine the criminality done in the war in Ukraine, we need a comparison to other wars, as the first and second world war. This is not especially the duty of medical research, but first medicine is always involved in wars, second the public in many countries is avoiding such comparisons, third as Kant argues “if justice perishes, it is no longer worth living on earth” (Figure 22), and fourth the Author’s families from the father’s side (Stettin) and mothers’ side (Regentin) were displaced persons (Figure 23), of which nobody wants to speak about.

In Figure 24 some data of World War II are summarized with respect to Germany. There were 15 million German refugees; 3.5 million were killed on their way. The most well-known case was the sinking of the “Gustloff” (Figure 25). 300 000 women were raped, abased, or killed. Seven million Germans starved on hunger or disease in between 1946-1947. Eisenhower forced German soldiers to dig ditches in wet ground (Rheinwiesen) to stay in and stopped the Red Cross to bring them food. One million German soldiers died in the hands of the USA after the war according to the statement of three persons of Figure 24.


Expulsion/refugees took place not only in the former east Germany (East Prussia, West Prussia, Silesia, east Pomerania (where the Authors father family is from), but also in middle Germany, as for example, around Dresden (Figure 20). Eisenhower, the head of US air force, gave the order for bombing. When refugees were running over the bridges of the river Elbe to escape, they were attacked by USA fighter pilots. In the war in Ukraine, it is distinguished whether military objects are attacked or refugees. Eisenhower’s ancestors had to leave Baden-Württemberg (Germany) in 1792. It could well be that Eisenhower had other motivations for bombing than to win the war.
It is therefore important that the USA is laid open all documents about World War two, what had not happened till now. One cannot understand why the Australian Julian Assange is treated that hardly. Is the USA afraid that a whistleblower may open the documents of World War two?
Many refugees, especially from East Prussia, their children and grandchildren are still suffering on the loss of their home country and culture. On the graves they sometimes have the former home country (Figure 26). When the Author drives with the car through the former East Germany (Figures 22 & 23), he suffers by seeing the familiar old houses, small streets surrounded by tries, the old rails of the train, the lakes, and hills, what also parents and grandparents talked about, when sitting in the evenings at the warm oven (Kachelofen).
Since the author has been a theoretical nuclear physicist [66], he must state the following. The physicists Heisenberg (see also collected works of Heisenberg, especially section C, volume 5, page 164); Einstein and Bohr had the agreement not to tell politicians about the possibilities of nuclear forces with respect to build an atomic bomb. But Einstein convinced Roosevelt in a letter to build the (first) atomic bomb. Following Hiroshima and Nagasaki, he felt sorry for that. The German Heisenberg did not build an atomic bomb for Hitler. After the war, Adenauer did not use the knowledge of Heisenberg to build nuclear power plants and Heisenberg (the father of Quantum Mechanics) was disappointed because his expert knowledge was not used. Who knows how the development of nuclear power plants would have gone with his knowledge? Not only had the Author made the experience that expert knowledge (for example human repair neurophysiology) is not of interest anymore.
- Schalow G (2013) Human Neurophysiology: Development and Repair of the Human Central Nervous System. Nova Science Publishers, Inc, Hauppauge NY, USA, pp: 734.
- Schalow G (2015) Repair of the Human Brain and Spinal Cord. Nova Science Publishers, Inc, Hauppauge NY, USA, pp: 525.
- Schalow G (2015) Neural network learning in human. Nova Science Publishers, Inc, Hauppauge NY, USA, pp: 324.
- Schalow G (2002) Stroke recovery induced by coordination dynamic therapy and quantified by the coordination dynamic recording method. Electromyogr. Clin Neurophysiol 42: 85-104.
- Schalow G (2002) Improvement after traumatic brain injury achieved by coordination dynamic therapy. Electromyogr. Clin Neurophysiol 42: 195-203.
- Schalow G, Jaigma P (2006) Improvement in severe traumatic brain injury induced by coordination dynamics therapy in comparison to physiologic CNS development. Electromyogr Clin Neurophysiol 46: 195-209.
- Schalow G (2019) Regeneration of the human spinal cord via coordination dynamics therapy. Peertechz Publications, eBook, pp: 97.
- Schalow G (2009) Partial cure achieved in a patient with near-complete cervical spinal cord injury (95% injury) after 3 years of coordination dynamics therapy. Electromyogr Clin Neurophysiol 49: 199-221.
- Schalow G (2002) Recovery from spinal cord injury achieved by 3 months of coordination dynamic therapy. Electromyogr Clin Neurophysiol 42: 367-376.
- Schalow G (2003) Partial cure of spinal cord injury achieved by 6 to 13 months of coordination dynamic therapy. Electromyogr Clin Neurophysiol 43: 281-292.
- Schalow G, Jaigma P, Belle VK (2009) Near-total functional recovery achieved in partial spinal cord injury (50% injury) after 3 years of coordination dynamics therapy. Electromyogr Clin Neurophysiol 49: 67-91.
- Schalow G (2010) Cure of urinary bladder functions in severe (95%) motoric complete cervical spinal cord injury in human. Electromyogr Clin Neurophysiol 50: 155-179.
- Schalow G (2021) CNS Repair in a Girl with a Spinal Cord Injury. Adv Pub Health Com Trop Med 121: 201-226.
- Schalow G (2006) Cerebellar injury improvement achieved by coordination dynamics therapy. Electromyogr Clin Neurophysiol 46: 433-439.
- Schalow G (2021) Cure-like brain-repair in a girl with atrophied cerebellum and pons through Coordination Dynamics Therapy. Adv Pub Health Com Trop Med 123: 1-47.
- Schalow G, Jaigma P (2005) Cerebral palsy improvement achieved by coordination dynamics therapy. Electromyogr Clin Neurophysiol 45: 433-445.
- Schalow G (2006) Hypoxic brain injury improvement induced by coordination dynamics therapy in comparison to CNS development. Electromyogr Clin Neurophysiol 46: 171-183.
- Schalow G, Pääsuke M, Ereline J, Gapeyeva H (2004) Improvement in Parkinson’s disease patients achieved by coordination dynamics therapy. Electromyogr Clin Neurophysiol 44: 67-73.
- Schalow G, Nyffeler T (2001) Koordinationsdynamik-Therapie: Myelomeningozele (Spina bifida).
- Schalow G, Nyffeler T (2000) Koordinatiosdynamik-Therapie: Skoliose.
- Schalow G (2019) Permanent coma patient re-learned to speak via Coordination Dynamics Therapy. Arch Clin Med Case Rep 3(2): 33-50.
- Schalow G (2017) Breast cancer growth inhibition via Coordination Dynamics Therapy. In: “Horizons in Cancer Research. Editor: Hiroto S. Watanabe. Nova Science Publishers, Inc, Hauppauge NY, USA, Vol 68; pp: 125-151.
- Schalow G (2020) Anaplastic oligodendroglioma WHO III brain cancer-patient recovered following operation, radiation and chemotherapy through Coordination Dynamics Therapy, which is also a Covid-19 treatment without ventilator. Int J Med Clin Imaging 5(2): 165-210.
- Christensen JF, Jones LW, Andersen JL, Daugaard G, Rorth M, et al. (2014) Muscle dysfunction in cancer patients. Ann Oncol 25: 947-958.
- Schalow G (2020) To live longer with a better quality of life through coordination dynamics therapy especially in patients with severe brain injury and brain-cancer. Int J Med Clin Imaging 5(2): 118-155.
- Schalow G (2021) Euthanasia in organ donation can be avoided through Coordination dynamics therapy. Adv Pub Health Com Trop Med 3: 1-34.
- Schalow G (2022) Basal ganglia and cortex repair through human-repair neurophysiology 12 years after hypoxia during birth. In Schalow G (Ed) Human Repair-Neurophysiology, BP International; pp: 181-227.
- Schalow G (2022) Spinal muscular atrophy repair through Coordination dynamics therapy and Translation of frog neuromuscular innervation pattern changes caused by neurotrophins to human. AOASM 2022(2): 1-81.
- Schalow G, Lang G (1987) Recording of Single Unit Potentials in Human Spinal Nerve Roots: A New Diagnostic Tool. Acta Neurochir 86: 25-29.
- Schalow G, Lang G (1989) Electrodiagnosis of human dorsal sacral nerve roots by recording afferent and efferent extracellular action potentials. Neurosurg Rev 12: 223-232.
- Schalow G, Zäch GA, Warzock R (1995) Classification of human peripheral nerve fibre groups by conduction velocity and nerve fibre diameter is preserved following spinal cord lesion. J Auton Nerv Syst 52: 125-150.
- Schalow G (2009) The classification and identification of human somatic and parasympathetic nerve fibres including urinary bladder afferents is preserved following spinal cord injury. Electromyogr Clin Neurophysiol 49: 263-286.
- Schalow G (2020) Classification and Identification of Human Peripheral Nerve Fibers by Conduction Velocity, Nerve Fiber Diameter and Natural Firing Patterns with Consequences for CNS Repair and Covid-19 Infection Treatment. Int J Med Clin Imaging 5(3): 231-314.
- Schalow G (1991) Oscillatory firing of single human sphincteric a2 and a3-motoneurons reflexly activated for the continence of urinary bladder and rectum. Restoration of bladder function in paraplegia. Electromyogr Clin Neurophysiol 31: 323-355.
- Schalow G (1993) Spinal oscillators in man under normal and pathologic conditions. Electromyogr Clin Neurophysiol 33: 409-426.
- Schalow G (1993) Action potential patterns of intrafusal g and parasympathetic motoneurons, secondary muscle spindle afferents and an oscillatory firing a2-motoneuron, and the phase relations among them in humans. Electromyogr Clin Neurophysiol 33: 477-503.
- Schalow G (2005) Phase and frequency coordination between neuron firing as an integrative mechanism of human CNS self-organization. Electromyogr Clin Neurophysiol 45: 369-383.
- Schalow G (2021) Phase and frequency coordination improvement among neuron firing for improved CNS self-organization and neural repair in Parkinson and spinal cord injury. Int J Med Clin Imaging 6(1): 350-425.
- Kelso JAS (1995) Dynamic Patterns. The Self-Organization of Brain and Behavior. MIT Press, Cambridge.
- Schalow G (2010) Scientific basis for learning transfer from movements to urinary bladder functions for bladder repair in patients with spinal cord injury. Electromyogr Clin Neurophysiol 50: 339-395.
- Haken H, Kelso JA, Bunz H (1985) A theoretical model of phase transitions in human hand movements. Biol Cybern 39: 139-156.
- Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, et al. (2004) Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron 42: 535-552.
- Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, et al. (1998) Neurogenesis in the adult human hippocampus. Nat Med 4: 1313-1317.
- Praag van H, Kempermann G, Gage H (1999) Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 2(3): 266-270.
- Guttmann L (1973) Spinal cord injuries. Comprehensive management and research. Blackwell, Oxford.
- Schalow G, Bersch U, Göcking K, Zäch GA (1995) Detrusor-sphincteric dyssynergia in paraplegia compared with the synergia in a brain-dead human by using the single-fibre action potential recording method. J Auton Nerv Syst 52: 151-180.
- Schalow G, Bersch U, Michel D, Koch HG (1995) Detrusor-sphincteric dyssynergia in humans with spinal cord lesions may be caused by a loss of stable phase relations between and within oscillatory firing neuronal networks of the sacral micturition center. J Auton Nerv Syst 52: 181-202.
- Schalow G (2022) Urinary bladder repair through Coordination dynamics therapy. Available online at: https://www.ics.org/2022/abstract/121
- Schalow G (2009) Coordination impairment between the somatic and parasympathetic nervous system divisions in the human sacral micturition center following spinal cord injury. Electromyogr Clin Neurophysiol 49: 337-367.
- Sperry RW (1945) The problem of central nervous system reorganization and muscle transposition. Quart Rev Biol 20: 311-369.
- Sperry RW (1947) Effect of crossing nerves to antagonistic limb muscles in the monkey. Arch Neurol Psychiat (Chicago) 58: 452-473.
- Weiss P, Brown PF (1941) Electromyographic study on coordination of leg movements in poliomyelitis patients with transposed tendons. Proc Soc Exper Biol Med 48: 384-387.
- de Groat WC (1975) Nervous control of the urinary bladder of the cat. Brain Res 87: 201-211.
- Torrens M, Morrison JFB (1987) The Physiology of the Lower Urinary Tract, Springer Verlag.
- Arbuthnott ER, Gladden MH, Sutherland F (1982) Autonomic innervation of muscle spindles from the hindlimb of the cat. Suppl Proc Roy Soc 17: 1.
- Barker D, Banks RW (1986) The muscle spindle. In: Engel AE and Banker, B.Q. (Eds.), Myology, Vol. 1, Part 1, McGraw-Hill, New York, pp: 309-341.
- Passatore GM, Fillipi GM, Grassi G (1985) Cervical sympathetic nerve stimulation can induce an intrafusal muscle fibre contraction in the rabbit. In: Boyd IA and Gladden MH (Eds.), The Muscle Spindle, Stockton Press, New York, pp: 221-226.
- Swash M, Fox KP (1985) Adrenergic innervation of baboon and human muscle spindles. In: Boyd IA and Gladden MH (Eds.), The Muscle Spindle, Stockton Press, New York, pp: 121-126.
- Hess WR (1981) Biological order and human society. In: Akert K (Ed.), Biological Order and Brain Organization - Selected Works of WR Hess, Springer-Verlag, Berlin, pp: 3-15.
- Schalow G (2006) Functional development of the CNS in pupils aged 7 to 19 years. Electromyogr Clin Neurophysiol 46: 159-169.
- Schalow G (2022) Human Repair-Neurophysiology, Book Publisher International, pp: 229.
- Zanone PG, Kelso JAS (1992) Evolution of behavioral attractors with learning: Nonequilibrium phase transition. J Exp Psychol Hum Percept Perform 18(2): 403-421.
- Yates FE (1980) Urodynamics and the study of female incontinence: the state of art as perceived by an outsider, with a modest proposal. In: Zinner NR, Sterling AM (Eds.). Female Incontinence. Prog Clin Biol Res 78: 1-14.
- Baehr M, Frotscher M (2005) Duus‘Topical Diagnosis in Neurology. Thieme Verlag, Stuttgart. (A very good book for medical neuroscience since it correlates structure and function in human nervous system injuries).
- Schalow G (2022) Continence Repair Through Coordination Dynamics Therapy. Adv Pub Health Com Trop Med 5: 1-97.
- Schalow G, Yamamura M (1971) Investigation of ground state correlation in an extended Lipkin-Meshkov-Glick-Modell. Nucl Phys A 161: 93-104.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Otolaryngology and Neurotology Research(ISSN:2641-6956)
- Chemotherapy Research Journal (ISSN:2642-0236)
- Journal of Allergy Research (ISSN:2642-326X)
- Journal of Carcinogenesis and Mutagenesis Research (ISSN: 2643-0541)
- Advance Research on Endocrinology and Metabolism (ISSN: 2689-8209)
- Journal of Oral Health and Dentistry (ISSN: 2638-499X)
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)
































