5890
Views & Citations4890
Likes & Shares
Malaria continues to rank as the number one parasitic scourge in the tropics accounting for over 50% of cases in hospitals. The ecology and diversity of the vectors play a vital role in the epidemiology of the disease. Identification of malaria vectors is essential in malaria mapping and modern protocols employ molecular techniques over morphometrics. The former being more definite and reliable in taxonomic studies. In this study, Anopheles and Culex species were identified using Polymerase Chain Reaction protocols. Mosquito larvae and pupae were collected via habitat evacuation from 33 breeding sites in 11 towns from Ifedore Local Government Area Ondo state, Nigeria. Mosquito larvae and pupae collected were reared to adulthood in the laboratory and identified morphologically to generic level using standard morphological keys. Morphologically identified Anopheles gambiae s.l. (340) Culex pipiens complex (150) were further subjected to Polymerase Chain Reaction protocol. Results showed that 148 (98.72%) of the Culex pipiens complex were identified as Culex pipiens quinquefasciatus while the Anopheles gambiae s.l. were identified as Anopheles gambiae s.s. (315), Anopheles arabiensis (21) and Anopheles merus (04). This study concludes that the residents of the area are at risk of mosquito-borne diseases, particularly falciparum malaria, whose vector was recorded in large numbers in the study area.
Keywords: Mosquito, Identification, PCR, Malaria, Vector, Transmission.
INTRODUCTION
Over 3,500 species of the family Culicidae have already been described comprising 43 genera [1], these figures are subject to continual change, as more species are discovered, and as DNA studies give reasons for restructuring and rearrangement of the family taxonomy [2]. Members of the family culicinae and anophelinae most especially the culicines and anophelines act as vectors of diseases. Separation of species is complicated by their confusing flexibility and probable gene flow between them. Because morphologically identical mosquitoes are often found in sympatry with other members of the same complex over much of their geographical range, their relative roles in the transmission of pathogens such as malaria, arboviruses and filariasis [3] and the ecological conditions, which determine their distributions [4-8] are often difficult to evaluate. Taxonomic identification of mosquitoes has been largely based on adult characters such as mating incompatibility developed in 1964 [9] differences in chromosome morphology developed in 1967 [10] isoenzymes protocols of 1978 [11] and recently DNA characteristics. The need for accurate method of identifying mosquitoes, most especially vectors is reflected by the fact that the basic taxonomy of the best-known vectors has remained contentious and has necessitated reinterpretation of the biology of vectors such as Anopheles gambiae complex. Some of the taxonomic complexity within An. gambiae s.s. has been elucidated through the analysis of chromosome inversion [12,13] hydrocarbons [14] and increasingly by the use of molecular methods to identify species, chromosomal forms (i.e., specific karyotypes) and geographical variants [15,16]. Emphasis and efforts are being directed to the development and application of molecular tools and Enzyme Electrophoretic Variations for the specific identification of insect-borne disease vectors transmitted by members of a sibling species complex. Currently combination of keys for morphological identification [17-19] and molecular techniques based on polymerase chain reaction protocols derived from the modified procedure (protocol using Pre-mix) established by Scott and others [20] is now being used across the globe, in Nigeria a few research works has been done following this procedure [4,21-27]. However, in-depth understanding of the type’s mosquito is needed. Identification of these species molecularly is novel in the study area.
MATERIALS AND METHODS
Study Area
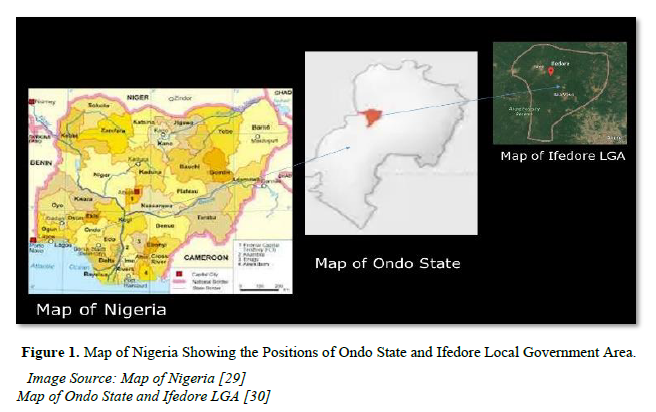
Ondo State is situated in the south western part of Nigeria with geographical coordinates of 7052ˈN, 6005ˈN and 5045ˈN, 4020ˈE. The state is bordered by Ekiti State in the north, Osun State by the west, Edo State at the eastern end, Ogun States State and the Atlantic Ocean in the southern area. Ifedore Local Government Area (5021ˈN, 5004ˈE) is one of the eighteen (18) Local Government Areas in the state and it is largely rural in nature with agrarian settlers (Figure 1). The Local Government is made up of eleven (11) towns and all were used for the study.
Mosquito Larval Collection and Rearing
Immature stages of mosquitoes were collected using standard plastic dippers, collection containers (each specific for a site, such sites included abandoned tyres, puddles, plastic containers and concrete holes) and transported to the laboratory. The larvae were reared and fed, with specially made feed comprising of yeast and biscuit in combination ratio of 1:5, to adulthood. The emerging adults were preserved in 1.5ml Eppendorf tubes containing silica gel.
Identification of Mosquito Species
All specimens were identified morphologically using Standard keys by [12,13,14] and after which Anopheles and Culex species were transferred in properly labelled vials to the Molecular Entomology and Vector Control Research laboratory of the Nigerian Institute of Medical Research, Yaba, Lagos, for molecular identification. All the reared Anopheles (n=340) and 156 of the reared Culex species (n=1270) were selected from each locality for molecular identification. DNA extraction was conducted with the aid of a genomic DNA extraction kit prep GEMTM insect (ZyGEM Corporation Limited, New Zealand). DNA was also extracted from the positive controls (no reference, positive controls are established mosquito DNA samples in the laboratory of Nigeria Institute of Medical Research) 1µl of the DNA extract from each mosquito sample was used as template for the Polymerase Chain Reaction synthesis. For the sister species identification of Anopheles gambiae complex, a standard protocol was adopted [15]. Master mix solution containing 2.5µl of 10x PCR reaction buffer, 2.5µl of dNTP, 1µl of MgCL solution, 2µl each of the other primers (Universal, Anopheles gambiae s.s., Anopheles arabiensis, Anopheles merus/melas), 4.9µl of sterile distilled water and 0.2µl Taq polymerase enzyme.
PCR for the 150 randomly selected Culicines was performed with universal and species-specific primers for the Culex pipiens Complex. Molecular identification of all the species complex was based on the species-specific nucleotide sequences in the ribosomal DNA intergenic spacers (IGS) following the modified procedure (protocol using Pre-mix) established by Scott and others [15]. The amplified DNA were separated on a 1.50% agarose gel stained with ethidium bromide and viewed on Ultra-Violet (UV) trans illuminator. Agarose gel with Tris-Borate Ethylene Di-Amino Tetraacetic Acid (TBE) Buffer: mixed and boiled in microwave until solution was cleared. Mixed between 1min heating to ensure that it does not boil over and cooled for 5 min. The master mix solution containing 4.00µl Pre-mix, 5.25µl ddH20, 1.00µl BSA (X1), 0.50µl ACEpip1, 0.50µl ACEpip2, 0.50µl B1246s and 1.00µl DNA (Figure 2).
The PCR machine was programmed for An. gambiae s.l., which consisted of one cycle of initial denaturation at 95°C for 2 min, followed by 30 cycles of denaturation at 95°C for 30s; annealing at 55°C for 30s; extension at 72°C for 40 ss and final extension at 72°C for 7 min (Hybridization and Extension phase), likewise the PCR machine was programmed for Culex pipiens complex, consisting of one cycle of initial denaturation at 94°C for 5 min, followed by 35 cycles of denaturation at 94°C for 30s; annealing at 58°C for 30s; extension at 72°C for 1min and final extension at 72°C for 5min.
12.5µl reaction volume of product was electrophoresed through 2.5% SEAKEM® agarose gel containing ethidium bromide and photographed under ultraviolet light illuminator. Photographs were taken under the Ultraviolet light inside the Ultra-Violet (UV) trans illuminator and results analyzed in comparison to standard wave length of each species namely: Culex pipiens pipiens 610 base pairs and Culex pipiens quinquefasciatus 274 base pairs, Anopheles quadriannulatus 153 base pairs, Anopheles arabiensis 315 base pairs, Anopheles gambiae s.s. 390 base pairs and Anopheles merus 464 base pairs.
RESULTS
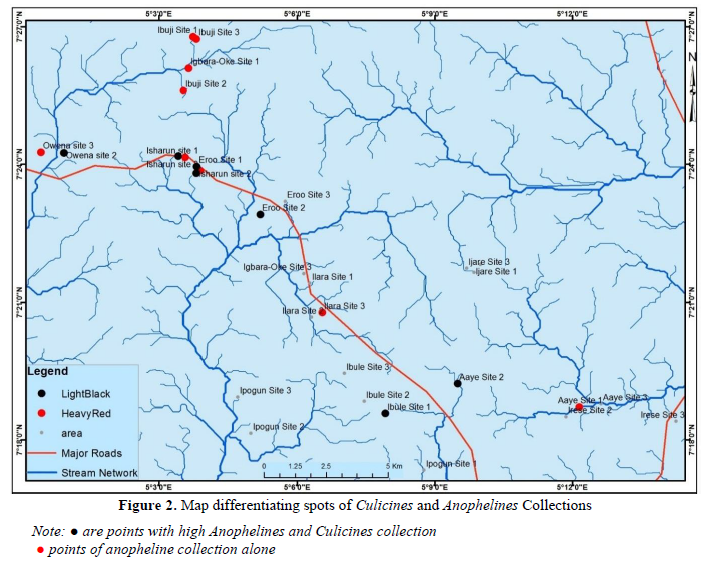
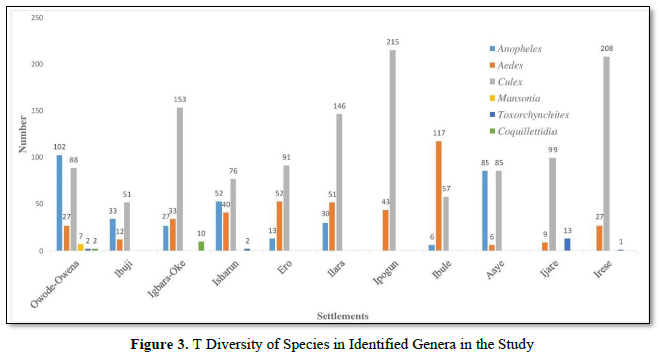
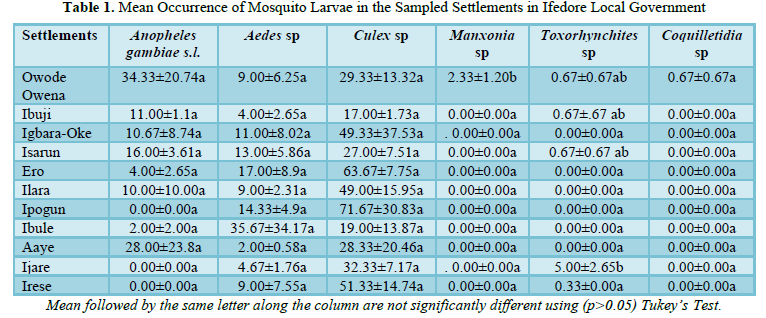
2051 imagoes reared from the 33 breeding sites were morphologically identified using the aforementioned standard keys. The identified mosquitoes were Culex species 1270 (740 males and 530 females; 61.92%), Aedes species 394 (248 males and 146 females; 19.21%), Anopheles gambiae s.l. 348 (194 males and 154 females; 16.97%), Toxorhynchites species 20 (14 males and 6 females; 0.98%), Coquillettidia species 12 (3 males and 9 females; 0.59%) and Mansonia species 7 (1 male and 6 females; 0.34%). The distribution of these species across the 11 localities in the Local Government Area is presented in (Figure 3). The mean occurrence of all the species is as presented in (Table 1) with significant difference only observed in two of the six species.
Of the 1270 Culex species mosquitoes identified morphologically as members of Culex pipiens complex, 150 were identified molecularly. and 148 were positive, it was noted that all the identified Culex species were Culex pipiens quinquefasciatus. The distribution of the molecularly identified Anopheles gambiae sensu lato across the Local Government Area is presented (Table 2).
DISCUSSION
After the morphological identification of mosquitoes from the Local Government Area, 6 genera were identified with Culex with the highest number of individuals as observed previously [8] Owode-Owena, Aaye, Igbara-oke, Isharun, Ilara and Aaye were the localities with Anopheles while the remaining 5 localities had other species the findings here are consistent with initial reports [5-7] that An. gambiae and An. arabiensis are extensively distributed throughout most of tropical Africa. The distribution of the male and female of the species in the Local Government Area, showed that number of male mosquitoes were more than the females except for Coquillettidia and Mansonia species, this would increase the tendencies of mating by the females.
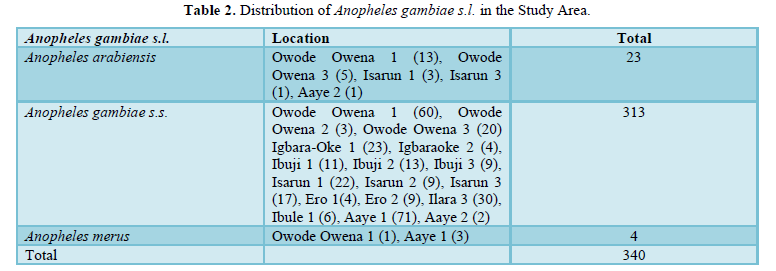
After selective molecular analyses, Culex pipiens quinquefasciatus was the only species of Culex pipiens complex encountered as also established by Yayocki and others [27]. Polymerase Chain Reaction protocols confirmed the presence of mosquitoes of importance to malaria transmission (Anopheles gambiae s.s., Anopheles arabiensis and Anopheles merus) and presence of Culex pipiens quinquefasciatus another vector of major public health importance. After PCR identification of the 340 Anopheles gambiae s.l., Anopheles arabiensis was 21, Anopheles gambiae s.s was 315 and Anopheles merus was 4. The record of Anopheles gambiae s.s. as the dominant species in this study corroborates literatures in parts of Nigeria and tropical regions that shares the same ecological characteristics as the study area [25,28]. An. merus was reported in low numbers in this research probably as result of the fresh nature of the water bodies. Due to cost of molecular analyses the other species were not subjected to analyses.
The occurrence of the mosquito species showed no significant difference across the villages except for Toxorhynchites species, which did not have wide distribution across localities. Toxorhynchites species whose larvae feeds on other mosquito larvae and can act as biological control agents were found in Owode-Owena, Ibuji, Isharun. The presence of the other species is very significant to public health in the study area, as they can act as vectors of various diseases ranging from Tularaemia, Arboviral diseases, such as yellow fever, Zika disease, dengue fever, and chikungunya, malaria and Lymphatic filariasis. Outbreak of these diseases in the area or neighboring local areas will have significant impact on the villagers, as the vectors are readily available.
CONCLUSION
The molecular work done in this research is novel in this locality, it will go a long way to help in controlling the species which are different in behavior, even to their sister species.
ACKNOWLEDGEMENT
The Authors acknowledges Master Sunday Ogunleye and Mr. Timilehin Oluwateru who helped on the field, staffs of Entomological and Parasitological Laboratory of the Nigeria Institute of Medical Research.
- Harbach RE (2004) The classification of genus Anopheles (Diptera: Culicidae): A working hypothesis of phylogenetic relationships. Bull Entomol Res 94: 537-553.
- Walter (2019) Walter Reed Biosystematics Unit. Retrieved: August 27, 2019. Available online at: http://www.si.edu
- Gatesnote (2015) Most lethal animal mosquito. Retrieved: December 12, 2015. Available online at: http://www.gatesnotes.com/Health/Most-Lethal-Animal-Mosquito-Week
- Awolola TS, Oyewole IO, Koekemoer LL, Coetzee M (2005) Identification of three members of the Anopheles funestus (Diptera: Culicidae) group and their role in malaria transmission in two ecological zones in Nigeria. Trans R Soc Trop Med Hyg 99: 525-531.
- Awolola TS, Oduola OA, Obansa JB, Chukwurah NJ, Unyimadu JP (2007) Anopheles gambiae s.s. breeding in polluted water bodies in urban Lagos, Southwestern Nigeria. J Vector Borne Dis 44: 241-244.
- Oduola AO, Olojede JB, Ashegbu CP, Adeogun AO, Otubanjo OA, et al. (2010) High level of DDT resistance in the malaria mosquito: Anopheles gambiae s.l from rural, semi urban and urban communities in Nigeria. J Rural Trop Public Health 9: 114-120.
- Oduola AO, Idowu ET, Oyebola MK, Adeogun AO, Olojede JB, et al. (2012) Evidence of Carbamate resistance in urban population of Anopheles gambiae s.s mosquito’s resistant to DDT and deltamethrin insecticides in Lagos, South-Western Nigeria. Parasit Vectors 5: 116-125.
- Gbaye OA, Afolabi OJ, Simon-Oke IA, Lasisi AO (2017) Abundance and spatial distribution of mosquitoes across three ecological zones of Ondo State Nigeria. Int J Mosq Res 4(5): 23-27.
- Davidson G (1964) The five mating types in the Anopheles gambiae Rivista di Malariologia 43: 167-183.
- Coluzzi M, Sabatini A (1967) Cytogenetic observation on species A and B of the Anopheles gambiae Parasitologia 9: 73-88.
- Miles SJ (1978) Enzyme variation in the Anopheles gambiae group of species (Diptera: Culicidae). Bull Entomol Res 68: 85-96.
- Coluzzi M, Sabatini A, Petraca V, Deco DMA (1979) Chromosomal differences and adaptations to human environments in gambiae complex. Trans R Soc Trop Med Hyg 73: 483-497.
- Coluzzi M, Petrarca V, Deco DMA (1985) Chromosomal inversion intergradation and incipient speciation in Anopheles gambiae. Bull Zool 52: 45-63.
- Milligan PJ, Phillips A, Broomfield G, Molyneux DH, Toure Y, et al. (1993) A study of the use of gas chromatography of cuticular hydrocarbons for identifying members of the Anopheles gambiae (Diptera: Culicidae) complex. Bull Entomol Res83: 613-624.
- Favia G, Torre AD, Bagayoko M, Lanfracotti A, Sagnon NF, et al. (1997) Molecular identification of sympatric chromosomal forms of Anopheles gambiae and further evidence of their reproductive isolation. Insect Mol Biol 6: 377-383.
- Favia G, Lanfrancotti A, Spanos L, Siden-Kiamos I, Louis C (2001) Molecular characterization of ribosomal DNA polymorphisms discriminating among chromosomal forms of Anopheles gambiae s.s. Insect Mol Biol 10: 19-23.
- Gillett JD (1972) Common Africa Mosquitoes and their Medical Importance with 48 color.
- Gillies MT, Coetzee M (1987) A supplement to the Anophelinea of Africa south of the Sahara (Afrotropical region). Publication of South African Institute for Medical Research, Johannesburg. 55: 1-143.
- World Health Organization (WHO) (2013) Malaria Entomology and Vector Control. Retrieve: May 22, 2017. Available online at: https://apps.who.int/iris/bitstream/handle/10665/85890/9789241505819_eng.pdf%3Bjsessionid%3D499D39C26B9EE5E5E260FF9D10DD6492%3Fsequence%3D1
- Scott JA, Brogdon WG, Collins FH (1993) Identification of single specimens of the Anopheles gambiae complex by polymerase chain reaction. Am J Trop Med Hyg 49: 520-529.
- Awolola TS, Hunt RH, Ogunrinade AF, Coetzee M (2002) Dynamics of the malaria-vector populations in coastal Lagos, South eastern Nigeria. Ann Trop Med Parasitol 96(1): 75-82.
- Awolola TS, Ibrahim K, Okorie T, Koekemoer LL, Hunt RH, et al. (2003) Species composition and biting activities of anthropophilic Anopheles mosquitoes and their role in malaria transmission in a holoendemic area of Southwestern Nigeria. Afr Entomol J 11(2): 227-232.
- Awolola TS, Oduola OA, Strode C, Koekemoer LL, Brooke B, et al. (2009) Evidence of multiple pyrethroid resistance mechanisms in the malaria vector Anopheles gambiae sensu stricto from Nigeria. Trans R Soc Trop Med Hyg 103: 1139-1145
- Oyewole IO, Ibidapo CA, Okwa OO, Oduola, AO, Adeoye GO, et al. (2010) Species Composition and Role of Anopheles mosquitoes in Malaria Transmission Along Badagry Axis of Lagos Lagoon, Lagos, Nigeria. Int J Insect Sci. 2010(2): 51-57. Retrieved: April 8, 2017. Available online at: http://www.la-press.com
- Obi OA, Nock IH, Adebote DA, Nwosu LC (2016) Ecological and molecular observations on Anopheles species (Diptera: Culicidae) breeding in rock pools on inselbergs within Kaduna State, Nigeria. J Mol Entomol 8(1): 1-10.
- Sule WF, Oluwayelu DO (2016) Analysis of Culex and Aedes mosquitoes in southwestern Nigeria revealed no West Nile virus activity. Pan Afr Med J 23: 116. Retrieved: November 14, 2017. Available online at: http://www.panafrican-med-journal.com/content/article/23/116/full/
- Yayock HC, Ndams IS, Kogi E, Ahmed AB, Vagime CG (2014) Distribution of mosquito species in Kaduna metropolis, Kaduna State, Northern Nigeria. Niger J Entomol 2014(special edn): 31.
- Emidi B, Kisinza WN, Mmbando BP, Mosha FW (2017) Effect of physico-chemical parameters on Anopheles and Culex mosquito larvae abundance in different breeding sites in a rural setting of Muheza, Tanzania. Retrieved: November 14, 2017. Available online at: https://www.researchgate.net/publication/317847438
- Rotowa OO, Olujimi JAB, Omole FK, Olajuyigbe AE (2015) Socioeconomic Factors Affecting Household’s Sanitation Preferences in Akure. Nig Eur Int J Sci Technol 4(5): 183-194.
- Google Earth (2019) Map of Ifedore Local Government Area. Retrieved: August 31, 2019. Available online at: https://earth.app.goo.gl/Ifedore
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Oral Health and Dentistry (ISSN: 2638-499X)
- Archive of Obstetrics Gynecology and Reproductive Medicine (ISSN:2640-2297)
- Chemotherapy Research Journal (ISSN:2642-0236)
- International Journal of Medical and Clinical Imaging (ISSN:2573-1084)
- Advance Research on Endocrinology and Metabolism (ISSN: 2689-8209)
- Journal of Pathology and Toxicology Research
- Journal of Psychiatry and Psychology Research (ISSN:2640-6136)





