Research Article
Existence Distribution and Habitat Characteristics of the Malaria Mosquito Larvae Anopheles Arabiensis And An. Sergentii in Lowlands of Tihamat Qahtan, Aseer Region, Saudi Arabia
6465
Views & Citations5465
Likes & Shares
Background: Targeting of vector control programmes is to interrupt the mosquito vector cycle specially the aquatic stages, which will reflect in the reduction of malaria vector abundance and subsequent disease transmission. This study was investigated the larval habitat succession and ecological parameters, which influence the larval abundance in Tihamat Qahtan (TQ), Asser Region, Saudi Arabia, hence act as indicators for evaluating the performance of malaria vector control operations in the study area.
Methods: Cross-sectional larval surveys were carried out from March 2006 to August 2007 in lowlands of TQ. Immature stages of mosquitoes were collected using standard dippers and identified using morphological-based keys. Environmental and physio-chemical characteristics of the aquatic habitats were measured onsite. Statistical analysis was performed.
Results: A total of 377 aquatic habitats was found positive for presence of anopheline larvae, a total of 4717 anopheline 3rd and 4th stage larvae were caught belonging to seven Anopheles spp. Clear and sun-exposed water bodies were more attractive for oviposition, wadi-related habitats were the most productive. An. sergentii larvae were found to occupy three different habitat types, and their population density was not significantly influenced by any of the studied ecological factors. An. d`thali and An. multicolor were the most abundant and exist in close association with malaria vector in TQ. A GIS-based survey strategy was developed.
Conclusion: The findings of this study suggest that implementation of effective larval control programme should be targeted the larval habitats succession information when larval habitats are fewer and manageable. Quantitative estimates of the contributions of various habitat types and their proximity to settlements provide a basis for planning a strategy for reducing malaria risk.
Keywords: Anopheles, Larvae breeding sites, An sergentii, An arabiensis, Mosquito, Aseer region KSA
INTRODUCTION
Globally, there were an estimated 229 million malaria cases in 2019 in 87 malaria endemic countries, declining from 238 million in 2000. At the Global technical strategy for malaria 2016-2030 (GTS) baseline of 2015, there were 218 million estimated malaria cases [1]. Saudi Arabia faces several challenges these will require a very strong disease and entomological surveillance system to implement focal vector control interventions, and effective border coordination [2]. Saudi Arabia reported only 38 indigenous malaria cases in 2019 [1]. Larval control is of paramount importance in the reduction of malaria vector abundance and subsequent disease transmission reduction Understanding larval habitat succession and its ecology in different land [3]. Although malaria control operations, particularly larviciding activities, had been extended to all malaria endemic areas in Saudi Arabia including Asser Region since 1963, information on the dynamics of the pre-adult stages of mosquito species is very scanty. Knowledge on the influence of habitat factors on larval production would be critical for understanding the spatial and temporal distribution patterns of the anopheline species [4]. However, the understanding of anopheline larval ecology in the region and elsewhere in Saudi Arabia, is limited and insufficient to achieve effective mosquito larval control [5]. A study carried out by Abdoon and Alshahrani [6]. Surveying mosquito larvae in some parts of Asser Region.
Larval control has been a key to vector control in areas in the attack phase of elimination and areas working to remain malaria-free and prevent the reintroduction of malaria. Interventions to reduce mosquito larval populations are a key component of Saudi Arabia’s malaria vector control and elimination programmes [7]. A clear system to overcome the constraints and ensure rapid and timely implementation of the activities was developed. More support and strength have been provided to those working in areas with Afro-tropical malaria. Currently, and since 2004, the MOH has adopted a new strategy of malaria elimination from the Kingdom. In addition to other antimalarial elements, control of larval stages of Anopheles species is the most promising control measure for malaria elimination. The present study conducted to determine the effects of environmental characteristics of larval habitats on distribution and abundance of anopheline larvae in lowlands of TQ, KSA and to recommend effective measures of larvae control.
MATERIALS AND METHODS
Study Area
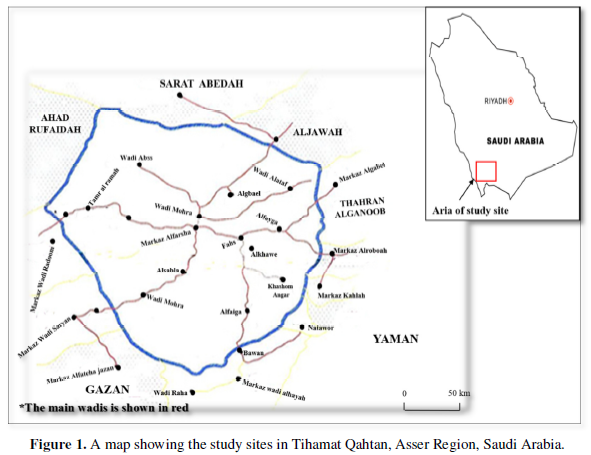
The survey was carried out in lowlands of TQ area (700 to 1800 m above sea level) (Figure 1). More than 13 main wadis run in the area, of total length of 425 km, about 228 km of this land has permanently running water of width ranges between 2-5 m in average. There are two rainy seasons, one coincides with the spring season (April - May) and the other in the autumn season (September - November), the average rainfall estimated to be 250 mm per annum [8].
Mosquito Larval Sampling
The larvae survey was conducted from March 2006 to August 2007. In each of 28 villages, a number of (10 - 15) aquatic habitats were randomly sampled for mosquito larvae (occurrence and density) between 10:00 am to 12:00 am. Active search was performed on monthly basis to identify and geo-locate potential larval habitats in the study sites. The presence and density of larvae were determined by dipping method [9] and from every habitat 10 dips were taken. The first two dips were collected in a white tray and the remaining 8 dips were sieved (Plate 1) to get rid of the water content and aggregate the larvae of the 8 dips into a net. The net with its larval content was immediately inverted onto the water surface of the first two dips to release the larvae and by this method we managed to avoid losses and obtained the whole larval content of each breeding site.
The white tray content of 10 dips of alive larvae of each breeding site was kept into a plastic bottle and transported to the Entomology laboratory at the Vector Control Administration, Abha, for processing and species identification.

Larval Habitat Characterizations
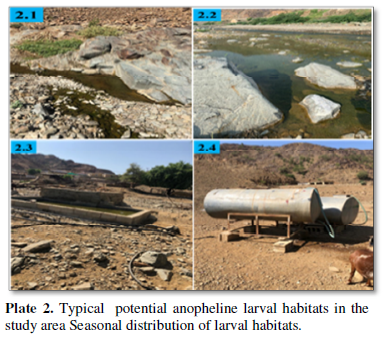
Aquatic habitats were defined as natural, e.g., wadi habitats (Plate 2.1 and 2.2) or man-made e.g., container or impoundment cement water pool (Plate 2.3) and household water tank (Plate 2.4). Water depth was measured by submerging the handle of the dipper (80 cm long) and estimated the depth; one third or less of the handle was considered as shallow water and more than one third was considered as deep water. A habitat exposed to direct sun light for the whole day was classified as sunny; a habitat located under shade for part of the day, classified as semi-shady, and that located under shade for the whole day classified as shady. Turbidity was measured by placing water samples in a glass test tube and holding it against a white background and classified as clear or turbid. Algae covering the habitat was measured as absent or present. Plant covering the habitat was measured as absent or present. Water temperature, pH and salinity were measured using its relevant electronic device HANNA (Hanna Instruments Inc., Germany). Water surface area was measured as less than 1 m3 and more than 1 m3. Distance to nearest house was measured as less than 100m and equals or more than 100 m.
Habitat Productivity
The productivity of the habitat was measured by calculating the number of larvae/dips among all study site.
Statistical analysis
Statistical analysis was performed for malaria vector species and the most predominant anopheline species. An analysis of variance (ANOVA) was used to measure the effect of season on population density and the effect of temperature and relative humidity on larval productivity.
RESULTS
Habitat Survey
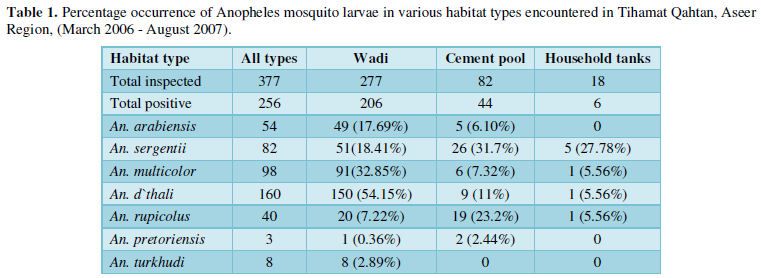
A total of 377 water bodies belonging to three types; wadi, cemented water pool and household water tank, were sampled for mosquito larvae during the study period in TQ. The most abundant habitat type was wadi that constituting more than (73.47%, N=277) of the total inspected areas. Of the total habitats examined, more than (67.9%, N= 256) were positive for anopheline larvae presence and about (80.47%, N= 206) were wadi habitat type (Table 1).
Habitat Productivity
As for species presence/prevalence, 'wadi' was the most productive for An. arabiensis (7.16 larvae per dip) compared to 'cement tanks' yielded 0.52 larvae/dip. Likewise, the highest larval density/dip for the most predominant anopheline species (An. d’thali and An. multicolor) were recorded from 'wadis' being 17.13 and 12.76 for the two species respectively.
Species Composition and Relative Abundance of Anopheline Larvae
A total of 4717 anopheline larvae of late instars were collected and seven anopheline species were identified
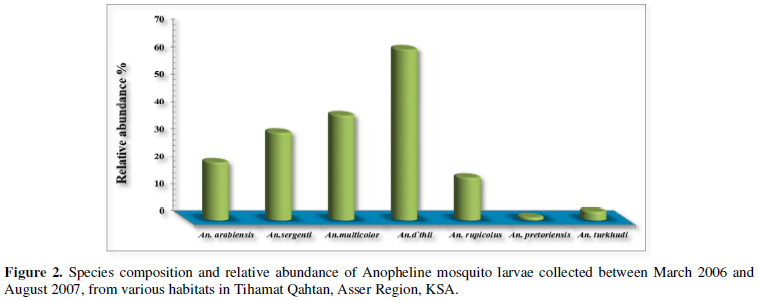
morphologically during the survey to occupy these habitats. Of these species, An. d`thali (34.62%, N= 1633) and An. multicolor (25.33%, N= 1195) were the predominant species, while other species were represented only in low percentages, including An. sergentii (15.65%, N= 738), An.
arabiensis (14.65%, N=691), An. rupicolus (9.03%, N=426), An. pretoriensis (0.42%, N=20) and An. turkhudi (0.30%, N=14) (Figure 2).
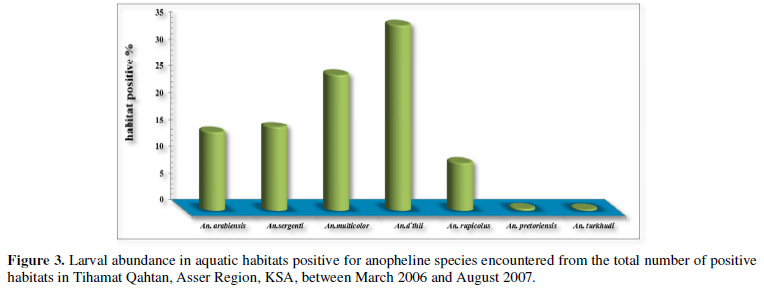


The larval abundance of positive habitats for each species is shown in (Figure 3). An. d`thali was the most abundant species in larvae positive habitats (62.5 %, N=160), followed by An. multicolor (38.28%, N=98), An. sergentii (32.03%, N=82), An. arabiensis (21.09%, N=54), An. rupicolus (15.63%, N=40), An. pretoriensis (1.17%, N=3) and An. turkhudi (3.13%, N=8).
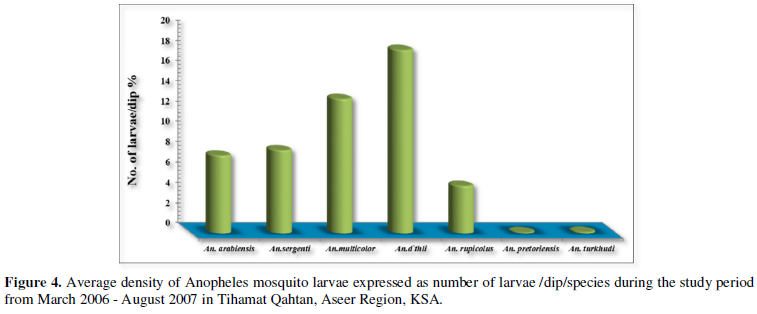
Figure 4 shows the average density of Anopheles mosquito larvae expressed as number of larvae /dip /species. Of the total collected larvae during the study period, An. d`thali presented the highest in larval density (18.14 larvae/dip), while An. arabiensis and An. sergentii, the known malaria vectors in the study area, revealed a density of 7.68 and 8.2 larvae/dip, respectively.
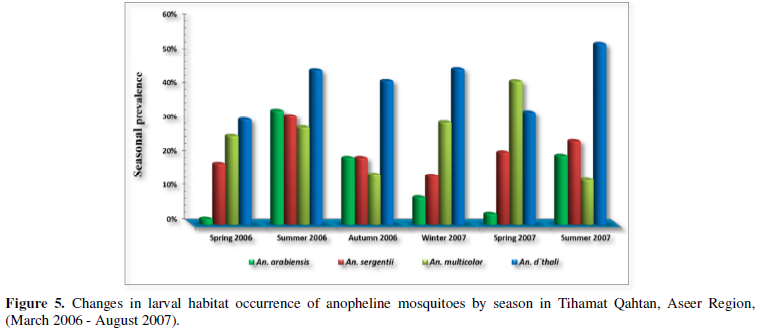
Four species of anopheline larvae, An. d`thali, An. multicolor, An. sergentii and An. arabiensis, were observed to occur in habitats during all seasons with variable degree of abundance (Figure 5). Habitats hosting An. d`thali larvae showed highest percentage occurrence during all seasons compared to other species, however, the difference between seasons was not significant (P = 0.091). Came next to this species was An. multicolor, that showed significant difference in their seasonal occurrence (P= 0.001). An. sergentii, although occurred during all seasons, while the difference between seasons was not significant (P= 0.233). An. arabiensis was the lowest in percentage of occurrence compared to other species, however, it showed significant different distribution between seasons (P= 0.000). Summer and spring seasons were most productive for An. arabiensis larvae, whereas, spring and winter seasons were productive for An. sergentii. The occurrence of An. d`thali was highest during summer season, whereas, percentage of occurrence of An. multicolor was highest during spring season.

Anopheline Species Larvae Density in Three Types of Habitats
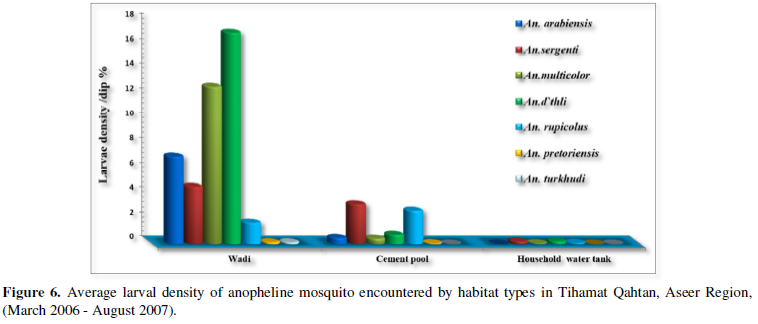
In Figure 6 results of average density of anopheline mosquito larvae in various habitats were summarized and expressed as the number of larvae of a certain species over the total number of a certain habitat type examined. Wadi habitats were found to be the most productive habitat, where about 83.72% (3949) of the total larvae were collected from this habitat, followed by cement pools 15.7%, (711). Whereas, household water tanks were found to produce 1.21% (57) of the total larvae collected, Wadi was the most productive habitat for An. arabiensis (7.16 larvae/dip) compared to cement pools (0.52 larvae/dip). As for An. sergentii the situation is different, where both types of habitats, wadi (4.68 larvae/dip) and cement pools (3.3 larvae/dip) were productive to this species. In sporadic cases, larvae of this species were found in household water tanks (0.22 larvae/dip). Although both An. d`thali and An. multicolor, the most predominant anopheline species, were collected from the three habitat types, they were found more in wadi habitats (17.13 and 12.76 larvae/dip, respectively).
Environmental Variables in Relation to the Occurrence and Density of Anopheline Larvae
The effect of environmental variables of habitats on the occurrence (attractiveness) and density level (productivity) of anopheline mosquito larvae was studied and results are summarized in (Table 2). Logistic regression analysis was detected some variables to be significantly correlated with the occurrence and productivity of anopheline larvae. Natural habitats, were more attractive and more productive to anopheline larvae than man-made ones (P = 0.000). Hence, wadi habitat, the natural habitat, showed highly significant attraction and productivity to anopheline larvae (P=0.000). Significant association was also observed in the occurrence of anopheline larvae in water bodies characterized by clear (P= 0.005) non-vegetated (P= 0.000) habitats. Most of these habitats were preferably sun-exposed (P= 0.005) with water temperature between 25 - 35°C (P= 0.004). Most of these habitats were more than 100 meters apart from the nearest houses (P= 0.001). High densities of larvae were observed in habitats more than 100 meters apart from the nearest inhabited houses (P= 0.001). Some other variables showed no significant association, including water depth (P= 0.065), water surface area (P= 0.247), occurrence of algae (P= 0.221), water pH (P= 0.125) and water salinity (P= 0.308). Significant association was found between high density production of anopheline larvae and sunny habitats compared to shady ones (P = 0.022). All other factors were tested for correlation coefficient between them and density levels of anopheline larvae did not exhibit any significant association.
Environmental Variables in Relation to the Occurrence and Density of An. Arabiensis Larvae
In Table 3 data showed the relation between measured environmental factors of water bodies and the occurrence and density of An. arabiensis larvae. All the twelve variables tested were significantly correlated with occurrence of An. arabiensis larvae except for shade (P= 0.321) and water pH (P= 0.25). Cement pools and household water tanks were much less likely to contain larvae of this species, whereas, wadis constituted by far, were the major habitat type for An arabiensis (P=0.009). Larvae of this species seem to prefer shallow water bodies rather than deep ones (P= 0.000). More than 76% of the habitats occupied by larvae of An. arabiensis were not vegetated (P= 0.003). Breeding sites with a surface area of 1 m3 or more was much more likely to contain An. arabiensis (P= 0.001). Turbid water diminished the chance that An. arabiensis larvae were present (0.002). Habitat s with salinity range of 1-2 were much more likely to.
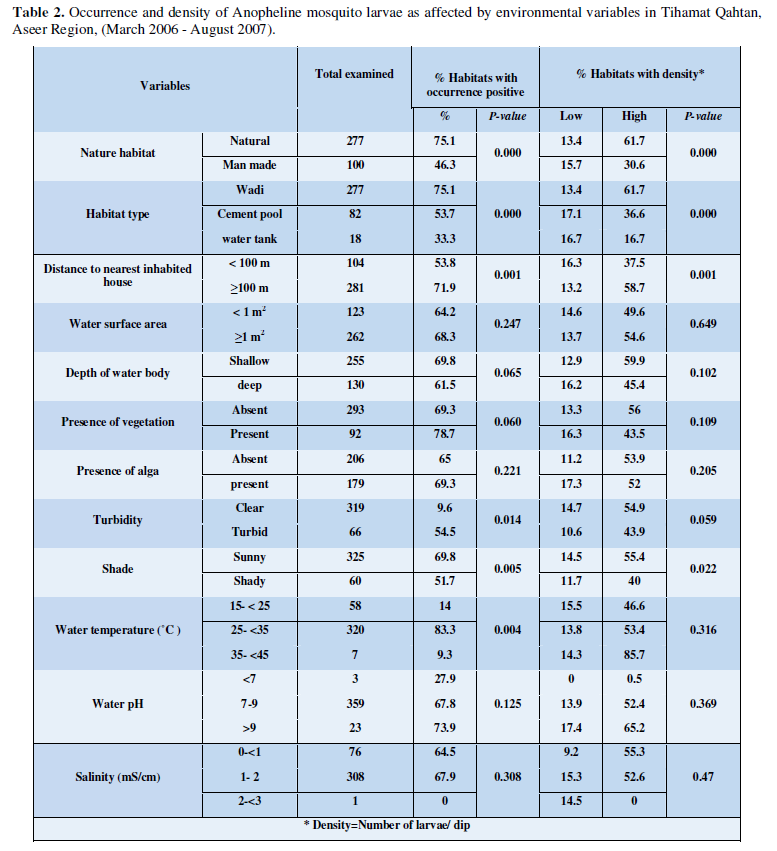
contain An. arabiensis larvae (P= 0.017). Most of the habitats surveyed were more than 1 meter apart from the nearest inhabited houses (73 %), hence An. arabiensis larvae were mostly collected from habitats at this location, showing statistically significant association (P = 0.018). An. arabiensis larvae also seem to prefer water habitats without algae (P= 0.005). The presence of An. arabiensis larvae seem to favour water temperature range of 25 - 35° C with slight significant association detected (P = 0.000). Larvae of An. arabiensis were rarely found in habitats with water pH more than 9, where only 6% of these habitats were occupied by this species, however, the association was not significant (P= 0.250). Exposure to sun light showed no significant association (P = 0.321) with water habitat. In general, the larval collection of An. arabiensis did not exceed 6% of the total mosquito larvae, despite this low collection, some variables exhibited statistically significant deference with regard to density of larvae. However, natural habitats with high density of larvae were significantly higher than man-made ones (P= 0.002). High densities of mosquito larvae were observed more in habitats lying more than 100 m from nearest houses (P= 0.024), also high densities were found in habitats with more than 1 m3 surface area (P= 0.005). Shallow water habitats induced the existence of high densities of An. arabiensis larvae compared to deep water habitats (P= 0.002). Moreover, high density of larvae was found in great association with habitats containing clear algae-free water (P= 0.002; P= 0.026, respectively). The pH values and water salinity of water habitats tested did not revealed any significant association with density level of An. arabiensis (P= 0.468 and P= 0.086 respectively).
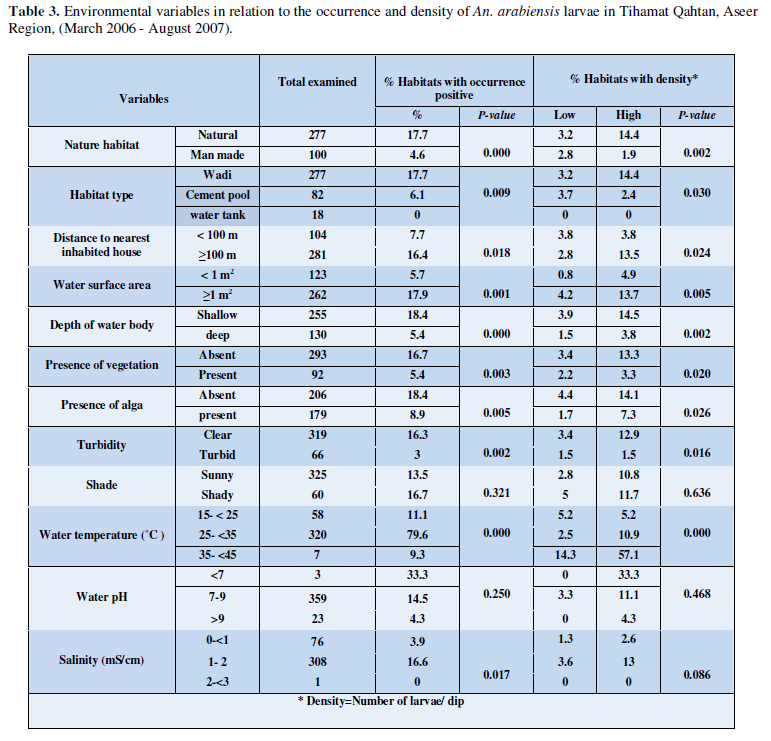
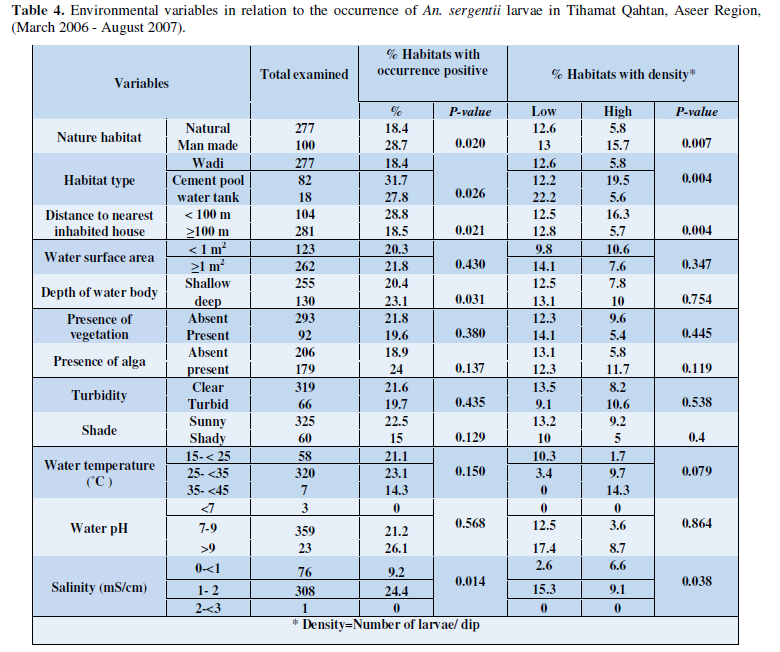
Environmental Variables in Relation to the Occurrence and Density of An. Sergentii Larvae
As shown in Table 4 An. sergentii larvae were found to occur in natural habitats as well as man-made ones, where larvae of this species were presented in 72% and 28% of the examined habitats, respectively.
Their larvae were collected from wadi, cement pools and household water tanks, however, a significant association was detected between them, where cement pool were found to be more associated with the presence of larvae of this species (P= 0.026). The most important variable that seems to represent significant association with An. sergentii larvae included distance of nearest inhabited houses (P=0.021). However, no significant association were observed between the occurrence of larvae of this species and other environmental variables. Habitats with high density of An. sergentii larvae were significantly observed in man-made habitats compared to natural ones (P= 0.007).
Of these man-made habitats, cement pools were found to host high densities of An. sergentii larvae than household water tanks and wadi habitats (P = 0.004). Also, high density of An. sergentii larvae were observed in habitats lying less than 100m apart from nearest houses (P = 0.004). Salinity range of 1-2 mS/cm was a significant factor for high density level of An. sergentii (P= 0.038). Other variables did not show any significant association with the larval densities of An. sergentii in various habitat types.
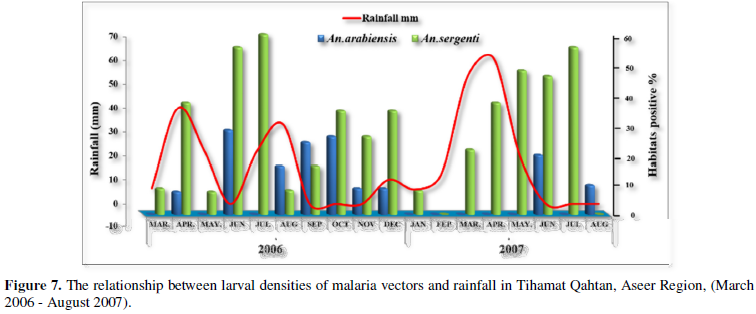
MONTHLY AND SEASONAL OCCURRENCE OF LARVAL POPULATIONS OF MALARIA VECTORS
The monthly fluctuation of mosquito larval habitat productivity has been exhibited in Figure 7 as a result of
rainfall inputs. During heavy rain falls, floods and dry months, larval habitat productivity of An. arabiensis was lowered. However, immediately post-rainy months showed increased larval habitat productivity. Other anopheline species studied did not show this phenomenon i.e., the larval habitat productivity didn't differ much with dry, rainy and post-rainy months. Statistical analysis was revealed significant differences in the larval habitat productivity of An. arabiensis between dry, rainy and post-rainy months, there was no significant difference in larval habitat productivity of An. arabiensis between dry months and rainy months (P= 0.984), whereas, significant difference was found between dry months versus post-rainy months and rainy versus post-rainy months (P= 0.000 and P= 0.001). As for An. sergentii, there was no significant difference between the dry, rainy and post-rainy months (P= 0.529, P= 0.322, P= 0.894 respectively).
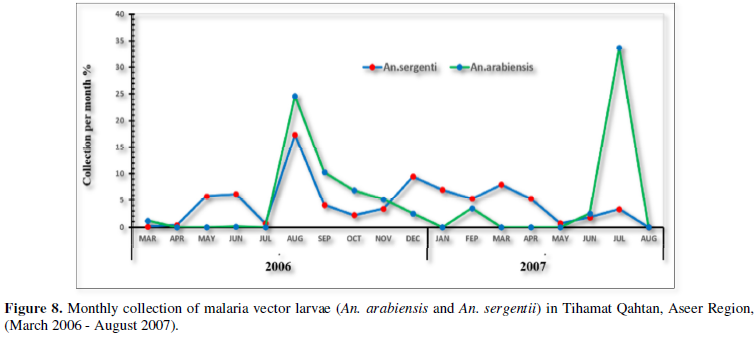
Data of Figure 8 showed the monthly productivity of An. arabiensis and An. sergentii that expressed as the percentage collection of larvae for each species in each month during the survey period. An. arabiensis showed only two peaks of larval productivity during the year, while An. sergentii showed three peaks. As for An. arabiensis, the high peak of larval productivity was observed in June and July (summer season) and the small peak was observed in December (winter season). Larval productivity of An. sergentii was observed during some months of the year; May, August, January and February, while their larval productivity declined to the minimum (no collection of larvae was obtained) during the rest of the months.

Data of Figure 8 showed the monthly productivity of An. arabiensis and An. sergentii that expressed as the percentage collection of larvae for each species in each month during the survey period. An. arabiensis showed only two peaks of larval productivity during the year, while An. sergentii showed three peaks. As for An. arabiensis, the high peak of larval productivity was observed in June and July (summer season) and the small peak was observed in December (winter season). Larval productivity of An. sergentii was observed during some months of the year; May, August, January and February, while their larval productivity declined to the minimum (no collection of larvae was obtained) during the rest of the months.
DISCUSSION
This study was conducted during continued pressure on mosquito larval population through year-round mosquito control program including; year-round larviciding interventions, seasonal residual house spraying and frequent space spraying operations in malarious areas of Asir Region in southwestern Saudi Arabia. Seven species of anopheline larvae were identified; of these An. arabiensis and An. sergentii were the two known vectors of malaria in Asir Region [6,10]. Wadi habitats were the most stable breeding places and supported year-round larval production in many areas regardless of the season. During the dry seasons, some of the wadis dry up, whereas, most of them contains narrow running water course with isolated pockets of stagnant water bodies at the edges (Plate 2.1). These pockets were mainly colonized by larvae of An. d`thali and An. sergentii. During the wet season, these wadis provide different types of breeding places rich with fresh water and encourage heavy breeding of An. arabiensis and An. multicolor (Plate 2.2). So, three to four days after the end of floods, the high productivity of larvae of the two species were observed. Cement pools are also a year around existing water bodies for mosquito to breed (Plate 2.3). Nevertheless, their productivity increases during the long dry seasons, during which larval habitats in wadis become scarce and the chemical characteristics of their water become non-attractive to certain species (e.g., An. arabiensis) for breeding. During the wet seasons, cement pools remain productive mainly to domestic mosquito species, like An. sergentii and An. d`thali. Household water tanks are kept at the house premises for human consumption (Plate 2.4). They are attractive only for An. sergentii.
One of the key objectives of this study was to detect ecological factors determining Anopheles spp. larval productivity and to characterize important breeding sites of anopheline mosquitoes and consequently, potential foci of malaria vectors. Nevertheless, An. sergentii had shown low larval population density during this study similar to what was observed by Abdoon and Alshahrani [6]. WHO also observed lower densities of adults of this species in Asir Region. This could be attributed to the long term and intensive larviciding application measures adopted throughout the year in the region. Beside this, also the high selectivity behaviour of their females for its favourable breeding habitat types, where they were found to select almost entirely, wadi pools of fresh water bodies formed after floods. In TQ, An. sergentii can be regarded as an important species, since it utilizes both natural (wadis) as well as man-made habitats (cement pools, house hold water tanks…etc.) for breeding purposed. Hence, malaria transmission potential in Aseer Region needs to be clarified, particularly under malaria elimination program currently adopted.
In the present findings, An. sergentii were also found in considerable densities in TQ, where the climate is characterized by moderate temperature and humidity, heavy rainfalls and many heavily flooded wadis most of the year. Recent reports on the existence of An. sergentii in TQ (Al-Farsha Province) was scanty, where only seven larval specimens were collected from a single site by Abdoon and Alshahrani [6]. During two years of larval search. However, in the present study, An. sergentii was collected as larvae from different sites of TQ area (Table 2). This might indicate a quick and wide spread occurrence of this species in TQ area as a result of more favourable climatic conditions. An. sergentii was described by Russell [11]. As an important vector of malaria in nine countries of the Eastern Mediterranean Region. An. sergentii is a known vector of malaria in oasis of Egypt and Libya [12,13] in Yemen also, strong indications were found to involve this species in malaria transmission [14].
The abundance of anopheline and culicine larvae are influenced differentially by ecological parameters [15]. The variability in larval population densities was explained by Grillet [16] and Gimnig and co-authors [17] to be due to spatiotemporal differences in food resources, predation pressure and the complex interaction between habitat factors such as water turbidity, depth, water temperature, water salinity and other similar factors.
Wadi habitats constituted by far the most productive habitat types for Anopheles mosquito larvae in the study area, and stand to be model mosquito larval habitat in TQ area. Similar findings were noted in some other Afro-tropical countries. In Kenya, Minakawa and colleagues [18] observed that Anopheles larval habitats were generally located near the valley bottoms or streambeds. Studies in Eritrea had similarly shown that mosquito breeding existed mainly at streambed pools [19]. These findings indicated that natural aquatic habitats located at the bottom or beds of wadis, valleys or streams are most attractive for female Anopheles to breed. This could be attributed to the fact that these running water courses always secure different kinds of water bodies after floods at their beds and bottoms that required by most of these species.
Three factors were found to influence the presence or absence of anopheline larvae in the study area. In clear water, larvae were much more likely to be present Bates [20], and Sattler and co-authors [15], were supported this finding, but Gimning and colleagues [21], found certain Anopheles spp. larvae (An. gambie s.l.) with increasing density, another study by Ye-Ebiyo and colleagues [22], found that the production of An. arabiensis was favoured in moderately turbid water while, excessive turbidity limited the production of larvae. Anopheles spp. larvae seem in this study, to avoid shady habitats while preferring habitats exposed to sun light. This finding disagrees with Keating and co-authors [23], who found that the proportion of water bodies with anopheline larvae presence versus absence was not significantly associated with shady and sun exposed habitats. Although, the occurrence of Anopheles spp. larvae appeared not to be affected by the presence or absence of algae in their habitats, An. arabiensis was found to prefer habitats without algae. This could be explained by the fact that An. arabiensis larvae survive more in fresh water habitats, mainly after rains or floods, before the development of algae in them. According to the statistical analysis water temperature, water pH and water salinity did not show any significant association to anopheline larvae presence or absence, indicating the low importance of these factors in determining the occurrence of larvae. Statistically significant association between the environmental variables and An. arabiensis occurrence was detected except for shade and water pH. This result indicated that An. arabiensis is highly selective in its habitat for breeding. Robert and researchers [24], found An. arabiensis larvae in wells with temperature range between 18-32°C, while in the present study the water temperature of habitats with An. arabiensis larvae was 25-35°C. Certain environmental factors studied influenced the productivity of An. arabiensis, while others have no significant effect on larval density. Moreover, some factors can significantly be associated with the presence or absence of this species, while the same factors have no significant effect on their density. These factors, as shown by this study, included the water salinity which was highly correlated with the presence of An. arabiensis (P= 0.017) while its effect on larval density was not significantly correlated (p = 0.086). The rest of factors measured excreted their significant effect on both the occurrence and density of larvae (Table 3). In conclusion, both shade and pH value of water body are not significant factors for determining the occurrence or density of An. arabiensis.
Three larval habitat types were found to contribute for mosquito productivity and distribution in the study area. This contribution varied greatly as a result of variation in habitat characteristics such as physical and chemical factors of their water bodies. Habitat type was found to be one of the key factors determining the presence of Anopheles larvae in Mwea, Kenya [25]. The most productive habitat type encountered during this study was wadis pool, where they comprised about 83.6% of total anopheline larvae collected. Besides that, this type of habitat hosted all the encountered species of anopheline larvae in the study area. An. arabiensis was found to rely mainly on wadi habitats for breeding. This can be explained by the fact that wadis secure, particularly during flood seasons, plenty of fresh water bodies with chemical and physical characteristics favored by this species.
Riverside flood pools were the most productive breeding sites for An. arabiensis as reported previously by Lewis [26] and Dukeen and Omer [27], in Northern Sudan. Similarly, Shililu [4] in Eritrea found that the highest densities of larvae of An. arabiensis were sampled from rivers and streams. Secondly were cement pool habitats, this habitat type was observed to be distributed almost in all houses in the villages of the study area, as they usually used it by the inhabitants for animal drinking and were built near their houses. Cement water pool are of different sizes and shapes. They are most productive habitat for certain species of Anopheles (e.g., An. sergentii, An. rupicolus). Other minor types of habitat included household water tanks which are used for reserving human drinking water, and always located inside houses, closely to the rooms, and data showed it to be an alternative habitat for An. d`thali and An. sergentii. Anopheline larvae were found colonizing more than 67% of the total examined aquatic habitat, indicating high larval productivity of this genus in the study area. While some larval habitat types were important in one zone, they were either absent or of only low significance in another zone at different changes in habitat characteristics that may affect larval development [19].
An. arabiensis, the principal vectors of malaria in TQ area Abdoon and Alshahrani, [6]. was found almost confined to these naturally occurring habitats (p= 0.000). In Eritrea, An. arabiensis production in the natural aquatic habitats such as streambed pools was high [19]. It was observed that during this study An. arabiensis productivity started to appear 2-3 days post-flooding of wadis. Unless another flood comes to wash these newly formed breeding places or a quick larviciding intervention, the outcome would be increased rate of productivity of this species, hence a mass flare up of adult mosquitoes would be expected in the affected area. An. arabiensis larvae were less likely to be present in other types of habitat and when so, they were found in very low densities. However, the importance of these habitats should not be underestimated because they might be the main source for maintaining An. arabiensis populations through the dry periods. Managing dry season larval habitats by continued pressure of larviciding interventions, would reduce the adult density during the immediate wet periods when the number of suitable aquatic habitats increases numerically and might eventually eliminate dry season An. arabiensis larval production [19]. Similarly, Dejene [28] in southwestern Ethiopia found that the Controlling the occurrence of mosquito larvae through larval should be conducted during the dry season, targeting the pools formed at the edge of the rivers as the water receded An. sergentii, a known secondary malaria vector in the study area, showed different breeding behaviour. Their larvae were found to be hosted by at least three different habitat types, namely, cement water pools, household water tanks and wadi habitats. However, cement pool habitat was the most preferable for their occurrence (p= 0.026), but larval harvest of this species among these habitats was significantly associated with household water tank (p= 0.004), indicating more chances for selection of a breeding habitat for their females. Out of these three habitat structures, household water tanks can be regarded as the most important since they are usually located inside houses in the vicinity of bedrooms. Also, deep water bodies with distance of less than 100 meters were more likely to attract An. sergentii females. However, the study showed that the population density of this species was not influenced by any of the tested factors such as; distance from/near houses and the salinity of water.
Both, An. d`thali and An. multicolor showed great tendency towards breeding in wadi habitats, P P< 0.000, respectively. They were always present in higher densities and close association with malaria vectors. Although they might play no role in malaria transmission in the study area, they can still be used for monitoring larviciding program effectiveness and as an early detection tool for insecticide resistance development in the area. Although An. d`thali, the most predominant anopheline species in the study area, larval productivity occurred in most habitat types, however, wadi habitats were nine times more significant in hosting this species larvae than all others collectively. Habitats colonized by An. arabiensis larval populations were found more frequently occupied by An. d`thali larvae, for more than 97% of An. arabiensis larvae positive habitats also hosted larvae of An. d`thali. Both species were also observed to demand at the same characteristics of certain environmental variables. An. multicolor was noted to breed rarely in habitats other than wadi. Only 6 out of 53 cemented pools were hosting larvae of this species, whereas none of the others did so.
The differences in the population level of mosquito immature between months can be attributed to the environmental variables that influence both the biology and the availability of resources in the habitats [29]. Rainfall periods and floods occurrence always leave behind fresh water pools devoid of vegetation or algae coverage. It was observed that the post rainy or flood months were the most productive months for An. arabiensis. Moreover, the air temperature and relative humidity during this time are all encouraging factors for female anopheline mosquitoes for high fecundity. So, during rainy season and flood months in Tihama Qahtan breeding activity of An. arabiensis maximized and wadi pool with their fresh water bodies attracted their females and high densities of their larvae were produced. This is an important determining factor which can be used during larviciding activities. Intensive efforts of larviciding application is needed to prevent the occurrence of high densities of this species during this season. However, during dry months where there were no floods or rains and the temperature was high, this type of habitats became changed to polluted with dense algae and vegetation coverage and not favoured by An. arabiensis larvae. During this dry period, the population of this genus disappear and their breeding was most probably directed towards a different type of habitat, which could not be detected during this period of the study. Hence, more elaborate study in this aspect and the discovery of the dry period breeding habitat type for An. arabiensis is of utmost importance. If such habitats are determined, they can give better chance to reduce An. arabiensis populations for the next generation. During the wet season, rain and floods secure many water surface areas and water pools and increase the chance for intensive larval productivity and high population densities of adults. During this period, it becomes extremely different to rely on larviciding measures for control of An. arabiensis larvae. So, it is advisable to attack larvae of this species during the dry season and by time and successive dry season attacks this policy would most probably lead to reduced population density to a level that incapable of maintaining seasonal malaria transmission. This control policy is successfully applied in the South Africa [30]. Gabrie [31] find that Presence of malaria mosquito larvae calls for immediate action and a vector control program should be conducted during the dry period, targeting drying streams An. sergentii has not yet proved to be an important vector for malaria in Aseer Region. However, their density distributional pattern, their breeding resting and feeding behaviour arise a question about their role in malaria spread. Hence, objective studies in their aspects to understand the exact role of these species is also demanding to strengthen more malaria control regime, particularly malaria elimination strategy. Therefore, it is highly recommended to define the breeding habitat utilized by females of An. arabiensis during the dry season that maintained their populations till the next breeding season.
CONCLUSION
The findings of this study suggest that implementation of effective larval control programme should be targeted the larval habitats succession information when larval habitats are fewer and manageable. The GIS-based survey strategy developed in this study provides key data on the population dynamics of An. arabiensis and An. sergentii. Quantitative estimates of the contributions of various habitat types and their proximity to settlements provide a basis for planning a strategy for reducing malaria risk by elimination of the vector population.
- World Health Organization (2020) World malaria report: 20 years of global progress and challenges. Geneva: World Health Organization. Licence: CC BY-NC-SA 3.0 IGO. Available online at: https://www.who.int/docs/default-source/malaria/world-malaria-reports/9789240015791-double-page-view.pdf?sfvrsn=2c24349d_5
- World Health Organization (2017) Regional malaria action plan 2016 - 2020 Towards a malaria free Region, Cairo: WHO Regional Office for the Eastern Mediterranean, 2017. Licence: CC BYNC-SA 3.0 IGO. Available online at: https://applications.emro.who.int/docs/EMROPUB_2017_EN_19546.pdf?ua=1
- Kweka EJ, Zhou G, Munga S, Lee M-C, Atieli HE, et al. (2012) Anopheline Larval Habitats Seasonality and Species Distribution: A Prerequisite for Effective Targeted Larval Habitats Control Programmes. PLOS One 7(12): e52084.
- Shililu JI (2001) Activity Report 111 Malaria Vector Studies in Eritrea. Environmental health project. Prepared for the USAID Mission to Eritrea under EHP Project 26568/E.X.ER.IMPLEMENTATION. pp: 1-44. Available online at: http://www.ehproject.org/PDF/Activity_Reports/AR111-EERMalVctStdFINAL.pdf
- Abdullah MA, Merdan AI (1995) Distribution and ecology of the mosquito fauna in the southwestern Saudi Arabia. J Egypt Soc Parasitol 25(3): 815-837.
- Abdoon AMO, Alshahrani AM (2003) Prevalence and distribution of Anopheline mosquitoes in malaria endemic areas of Asir Region, Saudi Arabia. East Mediterr Health J 9(3): 240-247.
- Ministry of Health (2019) Progress Toward Malaria elimination in the Kingdom of Saudi Arabia 2004 - 2015: A Success Story, King Fahd National Library Cataloging-in-Publication Data, L.D. no. 1440/7598. ISBN: 978-603-8209-48-6.
- Abdullah MA, Al-Mazroui MA (1998) Climatological study of the southwestern region of Saudi Arabia. I. Rainfall analysis. Climate Res 9: 213-223.
- O’Malley M (1989) Guidelines for larval surveillance. Proceedings of the Seventy-Sixth Annual Meeting of the New Jersey Mosquito Control Association, Inc. pp: 45-55.
- Ministry of Health MCP (1984) Malaria Control Programme in the Kingdom. Malaria Control Service, Saudi Arabia.
- Russell PK, Gould DJ, Yuill TM, Nisalak A, Winter PE (1969) Short report: Dispersal of Aedes aegypti in an urban area after blood feeding as demonstrated by rubidium marked eggs. Am J Trop Med Hyg 18: 580-583.
- Zahar AR (1974) Review of the ecology of malaria vectors in the WHO Eastern Mediterranean Region. Bull World Health Organ 50: 427-440.
- Kenawy MA (1995) Anopheles sergentii (Diptera: Culicidae): Seasonal variation in the development rates of immatures from El Faiyum and Siwa Oasis, Egypt. J Egypt Soc Parasitol 25(1): 257-268.
- Kouznetsov RL (1976) Distribution of Anophelines in the Yemen Arab Republic and its relation to malaria. World Health Organization, Geneva. Available online at: https://apps.who.int/iris/bitstream/handle/10665/65746/WHO_MAL_76.879.pdf;jsessionid=A578EA402EF84E2D2C25D82E06F3EFBD?sequence=1
- Sattler MA, Mtasiwa D, Kiama M, Premji Z, Tanner M, et al. (2005) Habitat characterization and spatial distribution of Anopheles sp. mosquito larvae in Dar el Salaam (Tanzania) during an extended dry period. Malaria J 4(1): 4.
- Grillet ME (2000) Factors associated with distribution of Anopheles aquasalis and Anopheles oswaldoi (Diptera: Culicidae) in a malarious area, northeastern Venezuela. J Med Entomol 37(2): 231-238.
- Gimnig JE, Ombok M, Otieno S, Kaufman MG, Vulule JM, et al, 2002. Density-dependent development of Anopheles gambiae (Diptera: Culicidae) larval in artificial habitats. J Med Entomol 39: 162-172.
- Minakawa N, Munga S, Atieli F, Mushinzimana E, Zhou G, et al. (2005) Spatial distribution of anopheline larval habitats in Western Kenyan highlands: Effects of land cover types and topography. Am J Trop Med Hyg 73(1): 157-165.
- Shililu J, Mbogo C, Ghebremeskel T, Githure J, Novak R (2007) Mosquito larval habitats in a semiarid ecosystem in Eritrea: impact of larval habitat management on Anopheles arabiensis Am J Trop Med Hyg 76: 103-110.
- Bates M (1949) The natural history of mosquitoes. New York: The Macmillan Company. Sci Educ 34(3): 208.
- Gimning JE, Ombok M, Kamau L, Havlett WA (2001) Characteristics of Larval Anopheline (Diptera: Culicidae) Habitats in Western Kenya. J Med Entomol 38: 282-288.
- Ye-Ebiyo Y, Pollack RJ, Kiszewski A, Spielman A (2003) Enhancement of development of larval Anopheles arabiensis by proximity to flowering maize (Zea mays) in turbid water and when crowded. Am J Trop Med Hyg 68: 748-752.
- Keating J, Macintyre K, Mbogo CM, Githure JI, Beier JC (2004) Characterization of potential larval habitats for Anopheles mosquitoes in relation to urban land-use in Malindi, Kenya. Int J Health Geogr 3(1): 9.
- Robert C, Richardson W, Elhassan IM, Giha H, Haviid L, et al. (1998) Seasonal changes in the Plasmodium falciparum population in individuals and their relationship to clinical malaria: a longitudinal study in a Sudanese village. Parasitology 116: 501-510.
- Muturi EJ, Mwangangi J, Shililu J, Muriu S, Jacob B, et al. (2007) Mosquito Species Succession and Physicochemical Factors Affecting Their Abundance in Rice Fields in Mwea, Kenya. J Med Entomol 44(2): 336-344.
- Lewis DJ (1949) The Extramination of Anopheles gambiae in The Wadi Halfa Area. Trans R Soc Trop Med Hyg 42(4): 393-402.
- Dukeen MY, Omer SM (1986) Ecology of the malaria vector Anopheles arabiensis Patton (Diptera: Culicidae) by the Nile in northern Sudan. Bull Entomol Res 76: 451-467.
- Getachew D, Balkew M, Tekie H (2020) Anopheles larval species composition and characterization of breeding habitats in two localities in the Ghibe River Basin, southwestern Ethiopia. Malar J 19: 65.
- Aditya G, Pramanik MK, Saha GK (2006) Larval habitats and species composition of mosquitoes in Darjeeling Himalayas, India. J Vector Borne Dis 43(1): 7-15.
- Sharp BL, le Sueur D (1996) Malaria in South Africa - the past, the present and selected implications for the future. S Afr Med J 86: 83-89.
- Dida GO, Anyona DN, Abuom PO, Akoko D, Adoka SO, et al. (2018) Spatial distribution and habitat characterization of mosquito species during the dry season along the Mara River and its tributaries, in Kenya and Tanzania. Infect Dis Poverty 7(1): 2.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Cancer Science and Treatment (ISSN:2641-7472)
- Journal of Pathology and Toxicology Research
- Journal of Otolaryngology and Neurotology Research(ISSN:2641-6956)
- Journal of Psychiatry and Psychology Research (ISSN:2640-6136)
- Chemotherapy Research Journal (ISSN:2642-0236)
- International Journal of Diabetes (ISSN: 2644-3031)
- International Journal of Internal Medicine and Geriatrics (ISSN: 2689-7687)










