Research Article
Multiple Adverse Drugs Effect in Pashtoon Patients with Helicobacter Pylori Infections in Pakistan
5498
Views & Citations4498
Likes & Shares
Background & Objective: H. pylori cause one of the most widespread and prevalent bacterial infection as the H. pylori resists the acidic environment of human stomach, so difficult to treat and eradicate. Treatment regiment contains multiple antibiotics, during therapy, multiple adverse drugs effect arises in patients with H. pylori infections. The current study was aimed to measure such effects in Pashtoon patients of our area.
Study design & setting: This Cross-sectional study was conducted in one (01) year period i.e., from January 2020 to December 2020 at District Headquarter Teaching Hospital, Bannu.
Sample Size: 89 out of 156 H. pylori infected patients were subject to combine triple therapy.
Methodology: Both serum antibody & stool antigen test positive patients were selected for current study at District, Bannu, KPK, and Pakistan. The regiment used for treatment for 14 days include antibiotics amoxicillin (1000 mg BD) 50 mg/kg/d, Clarithromycin (500 mg BD) 25 mg/kg/d & metronizole (400 mg BD) 20 mg/kg/d with PPI like omeprazole (40 mgOD) 2 mg/kg/d. Adverse drugs effects along with stool sample for to check eradication status were taken at second encounter after the completion of therapy duration.
Results: The current study showed that 71(78%) out of 89 patients had completed the therapy, treatment successes were in 57(80.2%) while treatment failure was in 14(19.8%) patients. The mean age and weight of patients was 34.0(±10.95), and 59.38 (±10.18) kg. Females were 54.92% as compare to male 45.07%. Male to female ratio was 1:1.2. Majority patients which showed adverse drug effects (ADEs) were married (71.84%), while 26.76% were single, showing statistically significant differences (X2=24.3, P=0.007 at p<0.05). Patients living in urban area were 61.97% & while, in rural area, it was 38.02%. ADEs shown by patients are complication like pain in stomach, long interval of meal 46.47%, pain during meal 23.94% and after meal 29.59 %showing significant differences (X2=28.2, P=0.002 at p<0.05).
Conclusion: Multiple antibiotics like amoxicillin, Clarithromycin/metronidazole along with proton pump inhibitor like omeprazole showed effectiveness against H. pylori eradication but most patients suffered adverse drugs effects. Our study showed that un-employed married females and patients living in urban area are more likely to suffer the adverse drugs effects than the others.
Keyword: ADE, H. pylori, Proton Pump Inhibitors, Amoxicillin, Bannu
INTRODUCTION
Helicobacter pylori being Gram-negative bacilli first isolated by Warren and Marshall in 1983 [1], has the ability to colonize stomach lumen of humans by its resistance to high acidic environment of stomach, cause the most widespread and prevalent bacterial infection that approximately infect two third of world population [2]. Helicobacter pylori is transmitted by the ingestion of bacilli and acquired orally within close contact families in childhood [3]. Colonization of these bacilli and if remain untreated induce chronic inflammation of underlying lumen mucosal surface and leads to prominent stomach abnormalities that include gastritis, peptic ulcer, gastric cancer and mucosal-associated lymph tissue lymphoma mostly account for upper gastrointestinal diseases [4,5].
World health organization recognize and categorize Helicobacter pylori bacilli as Class I carcinogenic bacterium of human beings that is most common in developing countries and mostly the infection occurs at early stage of life likely childhood but the majority of patients with infection does not have any clear clinically significant complications [6,7].
Several methodologies can be adopted for diagnosis of Helicobacter pylori beside common sign and symptoms; most commonly used methods are invasive and non-invasive methods. Invasive methods include endoscopy and biopsy for histological examination and non-invasive methods include serological, stool antigen test, urea breath test and molecular detection of Helicobacter pylori in samples from suspected patients [8], the common and easy to approach method considered to be non-expensive and accurate is non-invasive methods to be adopted [9]. Stool antigen test has been approved by United States Food and Drug Administration for detecting H. pylori before and after the therapy. Post treatment therapy antigen detection by SAT is growing in importance.
- pylori eradication is challenging amongst the multi drug users due to poor and incomplete medication of multidrug regiments due to adverse medication effects and often leads to failure of treatment. Other adherent reasons include sociodemographic changes of patients, gender, antibiotic resistance and the type of drugs used for eradication therapy [10]. But the most common reason of treatment and eradication therapy failure is adverse drugs effects that revealed poor adherence of patients to medications [11]. The current study based on post treatment antigen detection along-with adverse drugs effects. The stool antigen test was done both for primary detection as well as for eradication of H. pylori.
MATERIALS AND METHODS
- Study design and setting: Cross sectional study was conducted from January 2020 to December 2020, on positive pylori patients of combine triple therapy having 18-65 years of age at District Head Quarter Teaching Hospital, Bannu.
- Patients on combine triple therapy: The study individual was 89 patients those who were given combine triple therapy for 14 days and follow-up after treatment completion was done. The drugs regiments given was (amoxicillin, Clarithromycin/metronidazole/PPI), antibiotics like amoxicillin (1000 mg BD) 50 mg/kg/d, Clarithromycin (500 mg BD) 25 mg/kg/d & metronizole (400 mg BD) 20 mg/kg/d with a Proton Pump inhibitor, Omeprazole (40 mgOD) 2 mg/kg/d. No further treatment was given after the completion of 14 days.
- Inclusion & exclusion criteria: All the patients having both serum and stool antigen test positive were included in study while negative stool antigen test (SAT) and only serum positive and not willing patients were excluded from the study.
- Sample collection and lab Procedures: 3-5 ml blood was collected in Gel tube (Yellow tube) for pylori antibody test while stool sample was collected in sterile wide mouth container for Stool antigen test. The was serum separated from clot using centrifugation at 3000 rpm and was subject to Immunochromatic method (ICT) for detection of antibodies in patient’s serum as previously describes by Rehman [12]. The stool antigen test was done both for primary detection as well as for eradication of H. pylori for which One-step H. pylori Antigen Rapi-Card™ InstaTest was used to detect antigen in stool samples according to manufacturer protocol.
- Data Collection: Data at the time of recruitment (during first encounter) that includes patient’s sociodemographic and medical information and after the treatment for adverse medication/drugs effects was collected on pre-formed consented questionnaire. Stool samples were also collected during second encounter to determine the failure or success of eradication therapy of pylori.
- Ethical issue: Ethical approval was taken from concerned institutions. Patients were completely informed about their right to withdrawal any time from the current study.
- Statistical analysis: Latest statistical tools like SPSS statistical package and Microsoft office were used to evaluate and analyze the data for interpretations.
RESULTS
The study was conducted on 89 out of 156 patients, amongst 89 total patients 71(78%) had completed the combine triple therapy, 18 patients was dropped from study due to multiple reasons like non-willing and non-compliance with their treatment. The mean age (SD) of patients was 34.0 (±10.95) and range from 18 to 65 years of age where majority 72% was below age of 45; the mean weight of patients was 59.38 (±10.18) kg.
Table 1 of the study included socio-demographic information and factors and their association with adverse drugs effects, where Females were (39/71, 54.92%) as compare to male (32/71, 45.07%), Male to female ratio was 1:1.2, (X2=0.576, P=0.98 at p). Patients living in Urban area (44/71, 61.97%) and in rural area were (27/71, 38.02%, X2=2.97, P=0.704 at p
Majority patients were married (71.84%) while 26.76% were single (X2=24.3, P=0.007 at p<0.05). ADEs show by patients with complication like pain in stomach long interval of meal 46.47%, pain during meal 23.94% and after meal 29.59% (X2=28.2, P=0.002 at p<0.05). Both Marriage and stomach pain showed significant correlation with adverse drugs effects at 95% CI.
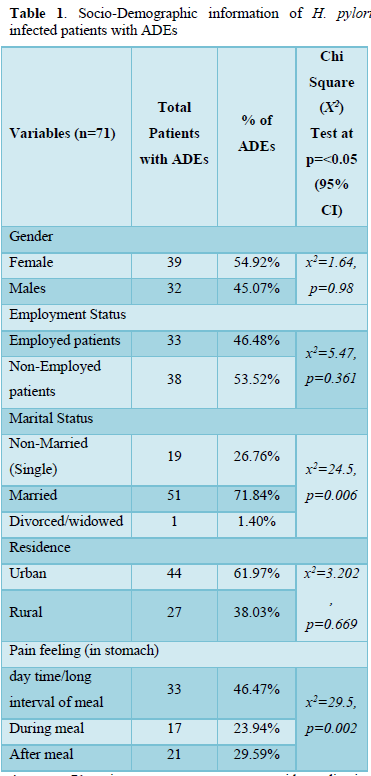
Majority patients were married (71.84%) while 26.76% were single (X2=24.3, P=0.007 at p<0.05). ADEs show by patients with complication like pain in stomach long interval of meal 46.47%, pain during meal 23.94% and after meal 29.59% (X2=28.2, P=0.002 at p<0.05). Both Marriage and stomach pain showed significant correlation with adverse drugs effects at 95% CI.

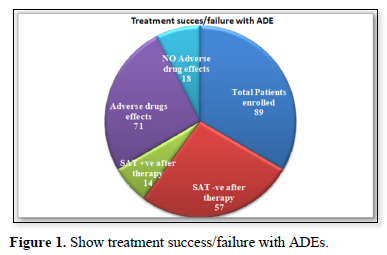
Amongst 71 patients, treatment outcomes with eradication checked by stool antigen test were 57(80.2%) while treatment failure with no eradication was 14(19.8%). Adverse drugs effects reported by patients was 71 (79.7% n=89) while 18 (20.3% n=89) does not show ADEs as shown by (Figure 1).


DISCUSSION
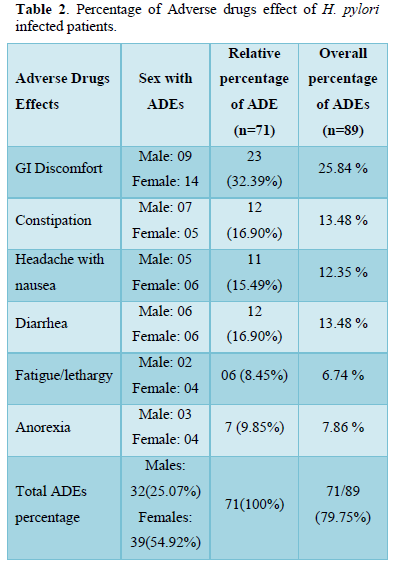
In the current study treatment outcomes reported on account of combine triple therapy for 14 days with regiment’s amoxicillin, Clarithromycin/metronidazole/PPI was quite much impressive, total patients 71(78%) has completed the combine triple therapy, amongst 71 patients, treatment outcomes with eradication checked by stool antigen test were 57(80.2%) similar to another study findings which were 78% [13], while treatment failure with no eradication was 14(19.8%). 14 days treatment with triple therapy was effective against H. pylori infection and our results were comparable to a study showed 87% successful eradication [14]. Adverse drugs effects reported by patients was 71 (79.7% n=89) while 18 (20.3% n=71) does not show ADEs. These ADEs were mild with no such serious condition of patients, most common effects recorded were 32.39% GI discomfort, constipation & diarrhea (16.90%), headache with nausea (16.3%), Anorexia 9.85% and fatigue/lethargy was (8.45 %) that match approximately the findings of Shakya [15] and Lee [16]. In another study by Masjedizadeh [15] the ADE reported was 76% approximately same as ours study which was 79.7% [17].
Our study uncovers patients with adverse drugs effects were more females (39/71, 54.92%) as compare to male (32/71, 45.07%), male to female ratio was 1:1.2, this might be due to differences in male to female physiological and anatomical figures like obesity and organ size that leads to more female as compare to male as like the other study [11,18] although no difference was made and was given the same dose to both genders. Mostly patients living in Urban area (44/71, 61.97%) as compare rural area (27/71, 38.02%) report adverse drugs effect as like the study of Gebeyehu [19]. In our study majority of patients with ADE were under the age of 45, Young married and unemployed adult patients report more adverse drugs effects than elderly patients that might be difference in physiological factors like marital status, excessive work conditions, socio-demographic differences etc.
Patients with long interval of meal at day time feel more pain and report ADE as compare to patients who report pain after the meal or during meal, our findings was comparable with the study of Sousa et al., 2018 which also reported more ADE during long interval of meals [20]. Stool antigen test was considered more effective while checking the eradication of H. pylori after treatment, in our study both prior to therapy and after treatment completion stool antigen test were performed that fully support our study.
CONCLUSION
Multiple antibiotics like amoxicillin, Clarithromycin/metronidazole along with proton pump inhibitor like omeprazole are effective against H. pylori eradication from infected individuals but most patients suffer adverse drugs effects that needs to be addressed beside the therapy. Overall eradication & treatment success was impressive. Married females, who are un-employed and patients living in urban area are more likely to be subjected to adverse drugs effect. Noninvasive method like Stool antigen test might be employed at every health facility prior to and after the treatment.
CONFLICT OF INTEREST
There was no conflict of interest.
- Warren JR, Marshall B (1983) Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 321(8336): 1273-1275.
- Mabeku LB, Ngamga ML, Leundji H (2018) Potential risk factors and prevalence of Helicobacter pylori infection among adult patients with dyspepsia symptoms in Cameroon. BMC Infect Dis 18(1): 278.
- Rowland M, Kumar D, Daly L. O′ Connor P, Vaughan D, et al. (1999) Low rates of Helicobacter pylori reinfection in children. Gastroenterol 117: 336-341.
- Walker MM, Crabtree JE (1998) Helicobacter pylori infection and the pathogenesis of duodenal ulceration. Ann N Y Acad Sci 859: 96-111.
- Labigne A, de Reuse H (1996) Determinants of Helicobacter pylori pathogenicity. Infect Agents Dis 5: 191-202.
- Chey WD, Leontiadis GI, Howden CW, Moss SF (2017) ACG clinical guideline: Treatment of Helicobacter pylori Am J Gastroenterol 112: 212-239.
- McNulty C, Owen R, Tompkins D, Hawtin P, McColl K, et al. (2002) Helicobacter pylori susceptibility testing by disc diffusion. J Antimicrob Chemother 49: 601-609.
- Ricci C, Holton J, Vaira D (2007) Diagnosis of Helicobacter pylori: Invasive and non-invasive tests. Best Pract Res Clin Gastroenterol 21(2): 299-313.
- Stefano K, Rosalia A, Chiara B, Federica G, Marco M, et al. (2018) Non-invasive tests for the diagnosis of helicobacter pylori: State of the art. Acta Bio Medica: Atenei Parmensis 89(Suppl 8): 58-64.
- Abbasinazari M, Sahraee Z, Mirahmadi M (2013) The Patients’ Adherence and Adverse Drug Reactions (ADRs) which are caused by Helicobacter pylori eradication regimens. J Clin Diagn Res 7(3): 462-466.
- Graham DY, Lew GM, Malaty HM, Evans DG, Evans Jr DJ, et al. (1992) Factors influencing the eradication of Helicobacter pylori with triple therapy. Gastroenterology 102(2): 493-496.
- Rahman SH, Azam MG, Rahman MA, Arfin MS, Alam MM, et al. (2008) Non-invasive diagnosis of H pylori infection: Evaluation of serological tests with and without current infection marker CIM. World J Gastroenterol 14(8): 1231-1236.
- Paz S, Florez Bracho L, Lasa JS, Zubiaurre I (2020) Helicobacter pylori Frequency of first-line treatment failure. Medicina 80(2): 111-116.
- Herardi R, Syam AF, Simadibrata M, Setiati S, Darnindro N, et al. (2020) Comparison of 10-day course of triple therapy versus 14-day course for eradication of Helicobacter pylori infection in an Indonesian population: Double-blinded randomized clinical trial. Asian Pac J Cancer Prev 21(1): 19-24.
- Shrestha SS, Bhandari M, Thapa SR, Shrestha R, Poudyal R, et al. (2016) Medication Adherence Pattern and Factors affecting Adherence in Helicobacter Pylori Eradication Therapy. Kathmandu Univ Med J 14: 58-64.
- Lee M, Kemp JA, Canning A, Egan C, Tataronis G, et al. (1999) A randomized controlled trial of an enhanced patient compliance program for Helicobacter pylori Arch Intern Med 159: 2312-2316.
- Masjedizadeh A, Zaeemzadeh N, Mard SA, Vanani GS (2015) Comparing the efficacy of four different protocols for eradicating of Helicobacter pylori infection in Ahvaz, southwest Iran. Prz Gastroenterol 10: 94-99.
- Burroughs VJ, Maxey RW, Levy RA (2002) Racial and ethnic differences in response to medicines: Towards individualized pharmaceutical treatment. J Natl Med Assoc 94(10 Suppl): 1-26.
- Gebeyehu E, Nigatu D, Engidawork E (2019) Self-reported adverse drug effects and associated factors among pylori infected patients on standard triple therapy: Prospective follow up study. PloS One 14(11): e0225585.
- Sousa LA, Fonteles MM, Monteiro MP, Mengue SS, Bertoldi AD, et al. (2018) Prevalence and characteristics of adverse drug events in Brazil. Cadernos de Saúde Pública 34: e00040017.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)
- Advance Research on Endocrinology and Metabolism (ISSN: 2689-8209)
- Journal of Allergy Research (ISSN:2642-326X)
- Journal of Ageing and Restorative Medicine (ISSN:2637-7403)
- International Journal of Diabetes (ISSN: 2644-3031)
- Journal of Pathology and Toxicology Research
- Journal of Carcinogenesis and Mutagenesis Research (ISSN: 2643-0541)



