Research Article
Mucin Expression Patterns of Human Colorectal Adenocarcinoma
3489
Views & Citations2489
Likes & Shares
Mucins are high-molecular weight epithelial glycoproteins with a high content of clustered oligosaccharides. We examined mucin expression including MUC1, MUC2, MUC5AC, HGM, and MUC6, as well as CD10 expression in human colorectal adenocarcinoma, and analyzed their clinicopathological significance. Immunohistochemically, colorectal cancer cases frequently expressed mucins and CD10 as follows: MUC1 in 58.8% (57/97), MUC2 in 56.7% (55/97), CD10 in 40.2% (39/97), MUC5AC in 15.5% (15/97), HGM in 19.6% (19/97) and MUC6 in 2.1% (2/97). Lymph node metastasis was frequently detected in MUC1-positive colorectal cancers, while lymphatic and/or venous invasions were frequently found in MUC5AC-negative and HGM-negative colorectal cancers. There were no apparent statistical correlations between clinicopathological features and expression of MUC2, MUC6 and CD10. Expression patterns of MUC1, MUC5AC and HGM are thought to become indicators of lymph node metastasis and lymphatic/venous invasions.
Keywords: Colorectal cancer, Adenocarcinoma, Mucin, Metastasis, Vascular invasion
INTRODUCTION
Mucins are high-molecular weight epithelial glycoproteins with a high content of clustered oligosaccharides O-glycosidically linked to tandem repeat peptides rich in threonine, serine, and proline [1]. There are two structurally and functionally distinct classes, i.e., secreted gel-forming mucins (MUC2, MUC5AC, MUC5B and MUC6) and transmembrane mucins MUC1 is a membrane-associated glycoprotein with a molecular weight of 290 kD [2]. Previous studies have demonstrated that MUC1 antigen is expressed in tumors of various human organs, and it has been shown to be an indicator of clinicopathological significance of carcinomas, such as breast, ovarian and renal cancers because it may inhibit cell-to-cell adhesion, leading to tumor metastasis [3-8]. On the other hand, the secreted gel-forming mucins (MUC2, MUC5AC, MUC5B and MUC6) are found in the gastrointestinal mucosal epithelia. The mucin expressions become immunohistochemical markers for cell differentiation, e.g., MUC2 for intestinal goblet cells, and human gastric mucin (HGM) for gastric foveolar/surface epithelium [9,10]. Recently, expression of secreted gel-forming mucins, such as MUC2 expression, has been reported as indicators of clinical significance in gastrointestinal malignancies.
Colorectal cancer is not only one of the most common cancers, but also one of the leading causes of cancer death in the Unites States, as well as Japan [11,12]. When it is discovered in its early stages, colon cancer is treated with surgery and often cured. However, many patients with colon cancer have no remarkable symptoms until the disease reaches an advanced stage, such as metastasizing to other organs. Recent advances in molecular biology have clarified multi-step carcinogenesis of the colorectal cancer, i.e., adenoma-carcinoma sequence [13-15]. On the other hand, mucin expression phenotypes of colorectal cancer have not yet been established.
In this study, we examined mucin expression including MUC1, MUC2, MUC5AC, MUC6, HGM, as well as CD10 expression in human colorectal adenocarcinoma, and will discuss their clinicopathological significance.
MATERIALS AND METHODS
Colorectal tissue specimens
Tissue samples were obtained from 95 patients with 97 colorectal cancer lesions (61 men and 34 women; mean age 67.1 ranging 41-88). The cases were surgically resected at Hirosaki University Hospital. Stages of colorectal cancer were classified according to the TNM classification [16].
Histological Examination
Colorectal tissue specimens were rapidly fixed in 10% buffered formalin for 24-48 h for histological and immunohistochemical analyses, and routinely embedded in paraffin. Tumor invasion was examined on sections 4 μm thick stained with hematoxylin and eosin as described previously [17,18]. Venous invasion was evaluated with elastica van Gieson staining.
Immunohistochemical staining
Immunohistochemical staining was performed using streptavidin biotin-peroxidase method with SAB-PO (M) kit (Nichirei, Tokyo, Japan). As primary antibodies, the following mouse monoclonal antibodies were used: MUC1 (Ma695, Novocastra Laboratories Ltd., Norwell, MA), MUC2 (Ccp58, Novocastra), MUC5AC (CLH2, Novocastra), MUC6 (CLH5, Novocastra), HGM (45M1, Novocastra) and CD10 (56C6, Nichirei). D2-40 (Dako Japan, Kyoto, Japan) immunostaining was also performed for evaluation of lymphatic invasion. Deparaffinized and dehydrated sections were pretreated with microwave heating at 98 degree C for 20 min for antigen activation. The sections were immersed in 0.3% hydrogen peroxide (H2O2) in methanol for 30 min to abolish endogenous peroxidase activities. Nonspecific bindings were abolished with diluted rabbit serum. Next, the sections were overlaid with primary monoclonal antibodies diluted with phosphate-buffered saline (PBS). The dilution magnification was set as follows: MUC1, MUC2, MUC5AC, MUC6 and D2-40 were diluted to 1:100; and HGM was diluted to 1:50. CD10 was already diluted for use. And these sections left over night at 4°C in a moist chamber. After being washed with PBS, the secondary biotinylated anti-mouse antibodies were applied for 20 min at room temperature. Then washed with PBS, the sections were treated with peroxidase-conjugated avidin for 20 min. The reaction products were visualized using diaminobenzidine tetrahydrochloride (DAB) action for 20-30 sec. Counter staining was performed using hematoxylin.
Evaluation of immunohistochemical staining
Maximum part of an excision in a colonic adenoma was evaluated as a representative section. As an evaluation of immunohistochemical staining, the ratio of the staining positive cells was measured with the light microscope. The section that presented mucin expression in the epithelial cells of 10% or more was judged as a positive case. These results were examined in statistics about the following parameter: histopathological type, depth of cancer invasion, venous invasion, lymphatic invasion, lymph node metastasis, liver metastasis, distant metastasis, peritoneal dissemination and TNM classification.
Statistical analysis
The 2 (chi-square) test were used for comparisons between group frequencies.
RESULTS
Immunohistochemically, colorectal cancer cases frequently expressed mucins and CD10 as follows: MUC1 in 58.8% (57/97), MUC2 in 56.7% (55/97), CD10 in 40.2% (39/97), MUC5AC in 15.5% (15/97), HGM in 19.6% (19/97) and MUC6 in 2.1% (2/97) of the colorectal adenocarcinoma tissues we examined (Figure 1). Mucin and CD10 expression were localized in the cytoplasm of the adenocarcinoma cells. Non-neoplastic colorectal mucosa showed MUC2 expression in the goblet cells, while MUC1, CD10, MUC5AC, HGM and MUC6 were not immunohistochemically detected in the non-neoplastic mucosa.
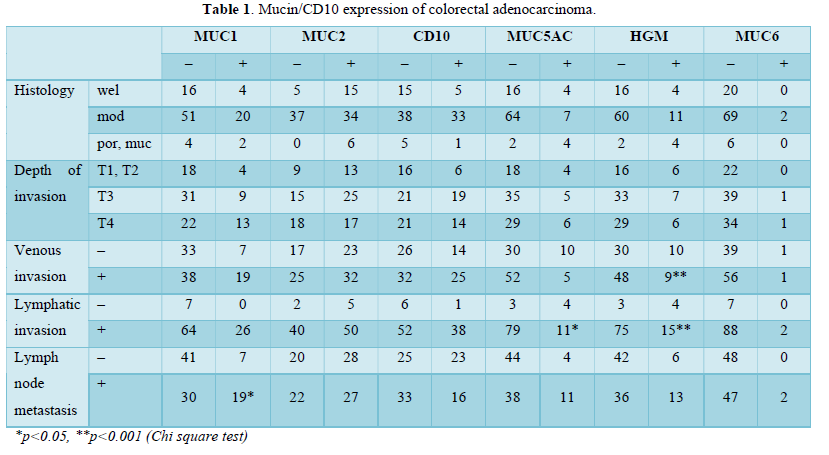
We analyzed the relationship between mucins/CD10 expression and clinicopathological features including pathological findings and TNM classification (Table 1). Venous and lymphatic invasions were evaluated with elastica van Gieson and D2-40 stainings, respectively. MUC1-positive colorectal cancers frequently showed lymph node metastasis (38.8%, p<0.05), compared with MUC1-negative cancers (14.9%). In addition, venous and/or lymphatic invasions were less frequently seen in the gastric-type mucins-negative cancers as follows: lymphatic invasion in 96.3% of MUC5AC-negative cancers, as well as lymphatic and venous invasion in 96.2% and 61.5% of HGM-negative cases, respectively. There were no apparent statistical correlations between clinicopathological features and expression of MUC2, MUC6 and CD10.
DISCUSSION
We examined immunohistochemical expression of mucins, as well as CD10, in human colorectal adenocarcinomas and analyzed their clinicopathological significance. This study demonstrated the relationship between various mucin expression and metastatic potentials in colorectal cancer patients. Lymph node metastasis was frequently detected in MUC1-positive colorectal cancers, while lymphatic and/or venous invasions were frequently found in MUC5AC-negative and HGM-negative colorectal cancers. There were no apparent statistical correlations between clinicopathological features and expression of MUC2, MUC6 and CD10.
Mucins are high-molecular weight epithelial glycoproteins with a high content of clustered oligosaccharides O-glycosidically linked to tandem repeat peptides rich in threonine, serine and proline [1].
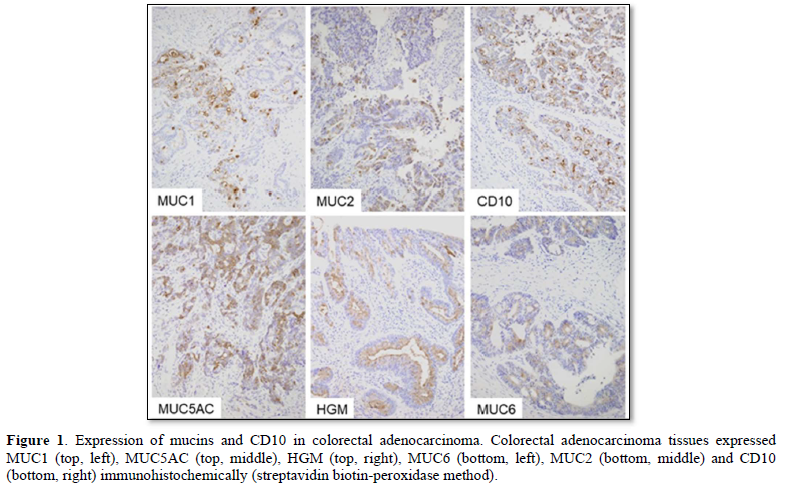

MUC1 (top, left), MUC5AC (top, middle), HGM (top, right), MUC6 (bottom, left), MUC2 (bottom, middle) and CD10 (bottom, right) immunohistochemically (streptavidin biotin-peroxidase method).

There are two structurally and functionally distinct classes, i.e., secreted gel-forming mucins (MUC2, MUC5AC, MUC5B and MUC6) and transmembrane mucins (MUC1, MUC3A, MUC3B, MUC4, MUC12 and MUC17), while some mucins (MUC7, MUC8, MUC9, MUC13, MUC15 and MUC16) do not categorized into either class. MUC1 has been shown to inhibit E-selectin-associated cell adhesion [19]. MUC1 includes cytokine receptor-like sequences in the extracellular region, and has been shown to function in signal transduction [20]. These observations suggested that MUC1 may function in tumor growth and metastasis of human carcinoma in vivo. Our present results demonstrated frequent lymph node metastasis in MUC1-positive cancer, and were supported by previous studies indicating that MUC1-mediated interactions may play important roles in tumor invasion [21-24]. More lymph node metastases in MUC1-positive cases suggested that MUC1-mediated mechanisms may cause carcinoma cells to reach lymph nodes through stromal lymphatic channels. On the other hand, the secreted gel-forming mucins (MUC2, MUC5AC, MUC5B and MUC6) are main mucin of normal gastrointestinal epithelia, and have been thought to play a role in the neoplastic progression and metastasis of gastrointestinal cancer [25-27]. HGM is the immunohistochemical marker for gastric foveolar/surface epithelium, and is encoded by MUC5AC core protein [28,29].
CD10 was originally used as a marker for common acute lymphoblastic leukemia antigen, but was shown to react with brush border of small intestine as well as germinal center of lymphoid follicles and microvilli of kidney [30-33]. According to the expression pattern of the aforementioned mucins (MUC2, MIC5AC, MUC6 and HGM), as well as CD10 expression, phenotypes of gastrointestinal epithelia are classified into the three groups; small intestinal type (CD10+, MUC2+, HGM/MUC5AC/MUC6+), large intestinal type (CD10-, MUC2+, HGM/MUC5AC/MUC6+), gastric type (CD10-, MUC2-, HGM/MUC5AC/MUC6+) and mixed type [(CD10+/-, MUC2+, HGM/MUC5AC/MUC6+) or (CD10-, MUC2+, HGM/MUC5AC/MUC6+)] [9,34]. Our present results demonstrated frequent lymphatic and/or venous invasions in MUC5AC-negative and HGM-negative colorectal cancers, i.e., intestinal type cancers, supported by previous studies [35]. Detailed mechanisms of vascular invasion in the MUC5AC/HGM-negative cancers have not yet clarified and should be analyzed in the future. However, no immunoreactivities of MUC5AC/HGM are thought to become an indicator of vascular invasion.
ACKNOWLEDGMENTS
This study was supported by JSPS KAKENHI, Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
- Byrd JC, Bresalier RS (2004) Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev 23: 77-99.
- Kufe D, Inghirami G, Abe M, Hayes D, Justi-Wheeler H, et al. (1984) Differential Reactivity of a Novel Monoclonal Antibody (DF3) With Human Malignant versus Benign Breast Tumors. Hybridoma 3: 223-232.
- Gatalica Z, Miettinen M (1994) Distribution of carcinoma antigens CA19-9 and CA15-3. Appl Immunohistochemistry 2: 205-211.
- Friedman EL, Hayes DF, Kufe DW (1986) Reactivity of monoclonal antibody DF-3 with a high molecular weight antigen expressed in human ovarian carcinomas. Cancer Res 46: 5189-5194.
- Ichige K, Perey L, Vogel CA, Buchegger F, Kufe D (1995) Expression of the DF-3-P Epitope in Human Ovarian Carcinomas. Clin Cancer Res 1(5): 565-571.
- Sagara M, Yonezawa S, Nagata K, Tezuka Y, Natsugoe S, et al. (1999) Expression of Mucin 1 (MUC1) In esophageal squamous-cell carcinoma: Its relationship with prognosis. Int J Cancer 84: 251-257.
- Fujita K, Denda K, Yamamoto M, Matsumoto T, Fujime M, et al. (1990) Expression of MUC1 mucins inversely correlated with post-surgical survival of renal cell carcinoma patient. Br J Cancer 80: 301-308.
- Mcguckin MA, Walsh MD, Hohn BG, Hohn BG, Ward BG, et al. (1999) Prognotic significance of MUC1 epithelial cucin expression in breast cancer. Hum Pathol 26: 432-439.
- Yao T, Tsutsumi S, Akaiwa Y, Takata M, Nishiyama K, et al. (2001) Phenotypic expression of colorectal adenocarcinomas with reference to tumor development and biological behavior. Jpn J Cancer Res 92: 755-761.
- Ho SB, Niehans GA, Lyftogt C, Yan PS, Cherwitz DL, et al. (1993) Heterogeneity of mucin gene expression in normal and neoplastic tissues. Cancer Res 53: 641-651.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA (2018) Global cancer statistics: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68: 394-424.
- Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS (2017) Colorectal Cancer Statistics, 2017. CA Cancer J Clin 67: 177-193.
- Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, et al. (1988) Genetic alterations during colorectal tumor development. N Engl J Med 319: 525-532.
- Hofstad B, Vatn M (1997) Growth rate of colon polyps and cancer. Gastrointest Endosc Clin North Am 7: 3345-3363.
- Bond JH (2000) Clinical evident for the adenoma-carcinoma sequence, and the management of patients with colorectal adenomas. Semin Gastrointest Dis 11: 176-184.
- Brierley JD, Gospodarowicz MK, Wittekind C (2017) Colon and Retum. In: TNM classification of malignant tumors, 8th John Wiley & Sons, Ltd West Sussex, UK, pp: 73-76.
- Yamamoto S, Kijima H, Hara T, Chino O, Shimada H, et al. (2005) Mucin expression and proliferating cell index of esophageal Barrett's adenocarcinoma. Int J Mol Med 16: 375-380.
- Kashiwagi H, Kijima H, Dowaki S, Ohtani Y, Tobita K, et al. (2001) MUC1 and MUC2 expression in human gallbladder carcinoma: A clinicopathological study and relationship with prognosis. Oncol Rep 8: 485-489.
- Utunomiya T, Yonezawa S, Sakamoto H (1998) Expression of MUC1 and MUC2 Mucins in Gastric Carcinomas: Its Relationship with prognosis of the patients. Clin Cancer Res 4: 2605-2614.
- Colomer R, Ruibal A, Genolla J, Salvador L (1989) Circulating CA15-3 antigen levels in non-mammary malignancies. Br J Cancer; 59: 283-286.
- Zhang K, Baeckstrom D, Brevinge H, Hansson GC (1997) Comparison of sialyl-Levis a-carrying CD43 and MUC1 mucins secreted from a colon carcinoma cell line for E-selectin binding and inhibition of leukocyte adhesion. Tumour Biol 18: 175-187.
- Wesseling J, van der Valk SW, Vos HL, Sonnenberg A, Hilkens J (1995) Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J Cell Biol 129: 255-265.
- Makiguchi Y, Hinoda Y, Imai K (1996) Effect of MUC1 mucin, an anti-adhesional molecule, on tumor cell growth. Jpn J Cancer Res 87: 505-511.
- Suwa T, Hinoda Y, Makiguchi Y, Takahashi T, Itoh F, et al. (1998) Increased invasiveness of MUC1 and cDNA-transfected human gastric cancer MKN74 Cells. Int J Cancer 76: 377-382.
- Betge J, Schneider NI, Harbaum L, Pollheimer MJ, Lindtner RA (2016) MUC1, MUC2, MUC5AC, and MUC6 in colorectal cancer: Expression profiles and clinical significance. Virchows Arch 469: 255-265.
- Niv Y, Rokkas T (2019) MCG Mucin expression in colorectal cancer (CRC): Systematic review and meta-analysis. J Clin Gastroenterol 53: 434-440.
- Reynolds IS, Fichtner M, McNamara DA, Kay EW, Prehn JHM, et al. (2019) Mucin glycoproteins block apoptosis; promote invasion, proliferation, and migration; and cause chemoresistance through diverse pathways in epithelial cancers. Cancer Metastasis Rev 38: 237-257.
- Bara J, Loisillier F, Burtin P (1980) Antigens of gastric and intestinal mucous cells in human colonic tumors. Br J Cancer 41: 209-221.
- Bara J, Gautier R, Mouradian P, Decaens C, Daher N (1991) Oncofetal mucin M1 epitope family: characterization and expression during colonic carcinogenesis. Int J Cancer 47: 304-310.
- Danielsen EM, Vyas JP, Kenny AJ (1980) A neutral endopeptidase in the microvillar membrane of pig intestine. Partial purification and properties. Biochem J 191: 645-648.
- Metzgar RS, Borowitz MJ, Jones NH, Dowell BL (1981) Distribution of common acute lymphoblastic leukemia antigen in nonhematopoietic tissues. J Exp Med 154: 1249-1254.
- Trejdosiewicz LK, Malizia G, Oakes J, Losowsky MS, Janossy G (1985) Expression of the common acute lymphoblastic leukemia antigen (CALLA gp100) in the brush border of normal jejunum and jejunum of patients with coeliac disease. J Clin Pathol 38: 1002-1006.
- Endoh Y, Tamura G, Motoyama T, Ajioka Y, Watanabe H (1999) Well-differentiated adenocarcinoma mimicking complete-type intestinal metaplasia in the stomach. Hum Pathol 30: 826-832.
- Yao T, Takata M, Tustsumi S, Nishiyama K, Taguchi K, et al. (2002) Phenotypic expression of gastrointestinal differentiation markers in colorectal adenocarcinomas with liver metastasis. Pathology 34: 556-560.
- Hazgui M, Weslati M, Boughriba R, Ounissi D, Bacha D, et al. (2021) MUC1 and MUC5AC implication in Tunisian colorectal cancer patients. Turk J Med Sci 51: 309-318.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Ageing and Restorative Medicine (ISSN:2637-7403)
- Journal of Carcinogenesis and Mutagenesis Research (ISSN: 2643-0541)
- International Journal of Diabetes (ISSN: 2644-3031)
- Journal of Otolaryngology and Neurotology Research(ISSN:2641-6956)
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)
- Journal of Pathology and Toxicology Research
- Advance Research on Alzheimers and Parkinsons Disease


