2650
Views & Citations1650
Likes & Shares
METHODS
A randomized, cross-sectional study was performed from 3 January to 17 February 2022. A questionnaire was utilized to collect information among the Palestinian population in the West Bank and Gaza Strip who received COVID-19 vaccines. A self-administered online survey (created with Google Forms) was circulated via social media platforms. We modified the adopted questionnaire developed to investigate the safety and side effects of COVID-19 vaccines [1]. The questionnaire was validated and translated into the Arabic language; i.e. the Arabic version was distributed to the Palestinian people.
The questionnaire used consisted of 44 questions and included three major parts in sequence, demographic data; clinical profile; and vaccine data. Participants were Palestinian citizens in the West Bank and Gaza Strips (≥18 years) who received at least one dose of any type of COVID-19 vaccine. A consent statement was included about voluntary participation and anonymity at the beginning of the questionnaire. Participants were directed to choose from a list of prespecified answers including (a multiple-choice design). Additionally, participants were asked to add any other side effects they might be experienced other than the ones mentioned in the answers.
Data collected were downloaded and entered into Microsoft Excel (Microsoft Corp., Redmond, WA). Descriptive statistics were computed for all variables using the Statistical Package for the Social Sciences (SPSS) (IBM Corporation, Armonk, NY, USA), software version 25. Chi-square analyses were performed to determine whether there are any statically significant differences between the expected frequencies of the side effects of COVID-19 vaccines used and the different recipients' variables. Results were analyzed to determine the safety, the potential side effects, and the perceptions of COVID-19 vaccines used in Palestine. Subsequent comparisons were made between participants' characteristics and the adverse effects of COVID-19 vaccines to find if there is a link between them. For quantitative variables, descriptive statistics were used and were expressed as mean, standard deviation or frequencies, and percentages. The exact test was performed as necessary for comparing categorical results, p ≤ 0.05 was considered for all statistical tests (see supplementary data).
RESULTS
Of 412 responses, 387 participants (132 males and 255 females) were included in the analyses. Twenty-five participants who gave incomplete responses were excluded. Most of the participants were from Hebron Governorate (n= 309, 79.8%), with an average age ranging from 20-29 years old. The majority of the participants (n= 269, 69.5%) had completed a bachelor's degree, and half of them were married (n= 196, 50.6%). The majority of respondents were non-smokers (n= 323, 83.5%), 40.8% (n= 158) lives in cities while 56.6% (n= 219) lives in villages, and 41.1% (n= 150) had income less than US$ 650). Only 9% (n= 35) work in the medical field, while 17.3% (n= 67) were government employees, and 38% (n= 147) were students. Most of the participants either have an O+ blood group (n= 115, 29.7%) or an A+ group (n= 116, 30%). The majority of the persons in the studied sample (n= 293, 75.7%) were not suffering from any chronic disease and most of them (n= 355, 91.5%) were also not suffering from an allergy to any types of foods or medicines (Table 1).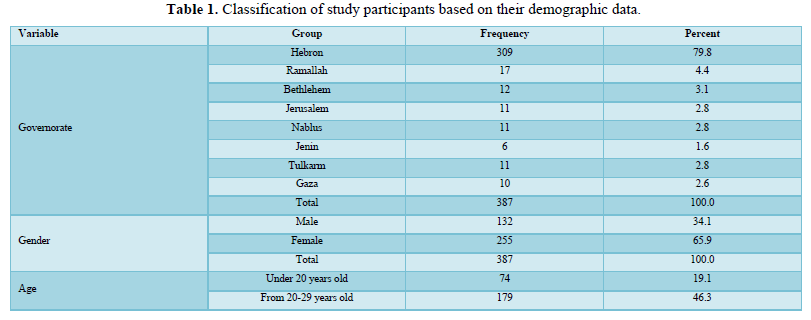
Most of the participants indicated that it is important to get a vaccine to protect themselves and others from COVID-19 as shown in Table 2. The degree of acceptance is high according to the participants, as 16.5% of the participants strongly agreed to receive and 50.4% agreed, while 25.6% were neutral, only 4.4% disagreed and 3.1% strongly disagreed to receive the vaccine. Thus, the participants have a high degree of getting a vaccine to be protected from the disease since the mean is 3.73 > 3.66 (which means that it is high according to the study Scale)*.
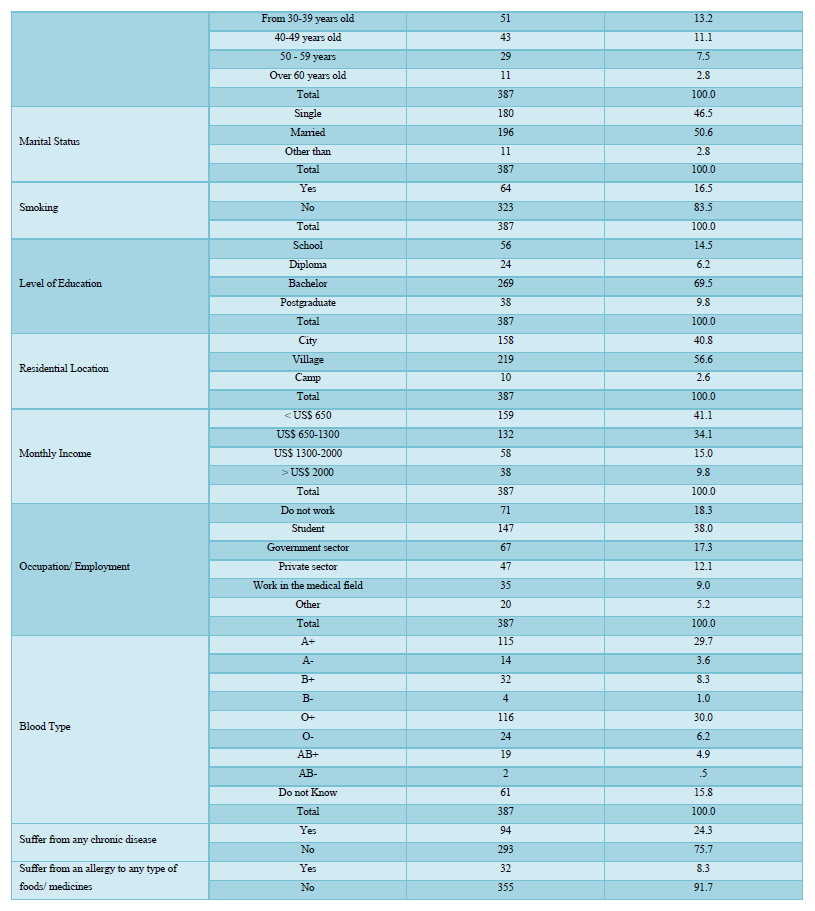
About half of the participants (n= 207, 53.5%) weren’t infected with COVID-19 viruses, while 85 individuals (22%) were infected before vaccination, 11 (2.8%) were infected after the vaccination, and 84 (21.7%), didn’t know if they have been infected or not Figure 1A. Besides about half (n=197, 50.9%) of the participants also were scared of receiving vaccines (Figure 1B). It was found that the sources of information about COVID-19 vaccines for the respondents were as follows: social media platforms (n= 138, 35.7%), and government-owned media platforms (n= 113, 29.2%) was the main source for COVID-19 vaccine-related information, while scientific and medical websites were a reasonable source too (n= 95, 24.5%). Few of the respondents (n= 33, 8.3%) get their information from friends and relatives, and only 8 (2.1%) have no information regarding COVID-19 vaccines (Figure 1C).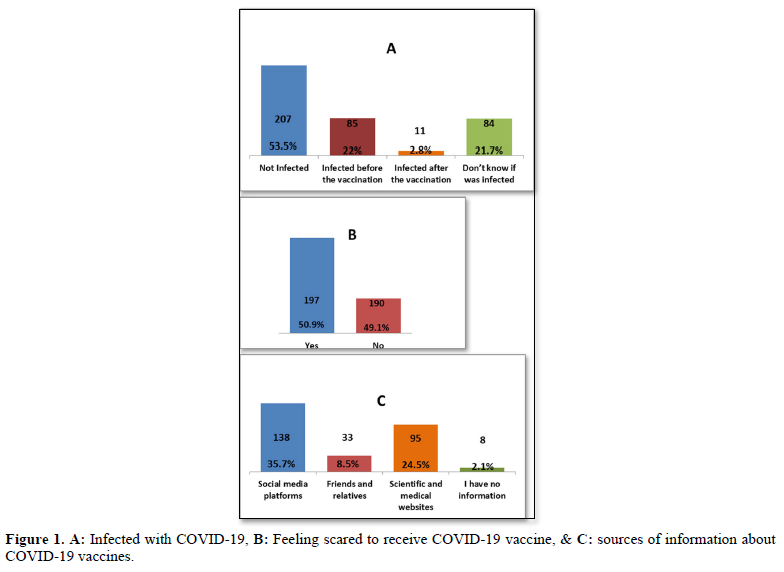
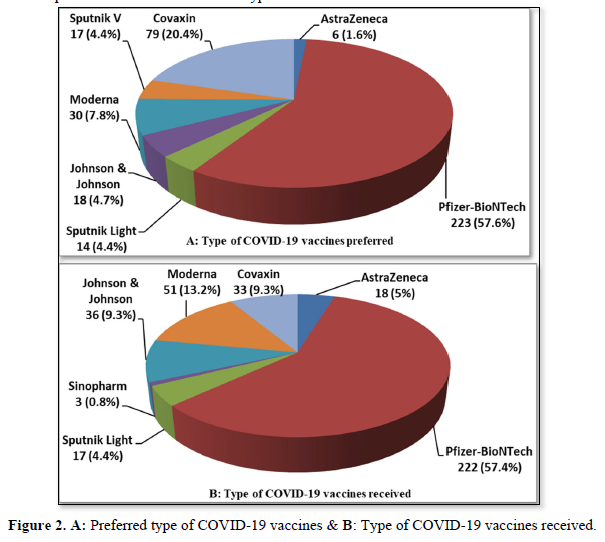
Figures 2 (A & B) shows the participants' frequencies and percentages of the type of COVID-19 vaccines they preferred to get and that they have received respectively. Before vaccination, the Pfizer-BioNTech- USA vaccine was the preferred vaccine among the precipitants if they have to choose the vaccine they wanted. Two hundred twenty-three (57.6%) of the respondents preferred to get the Pfizer vaccine, then 79 (20.4%) Covaxin- India vaccine, then Moderna- USA (n= 30, 7.8%), and only 6 participants (1.6%) preferred to have AstraZeneca/Oxford- UK vaccine, this might be due to published side effects of this type of vaccine. No one preferred to get the Sinopharm-China vaccine, while only a few preferred to get Johnson & Johnson-USA (n=18, 4.7%), Sputnik V (n= 17, 4.4%), or Sputnik Light- Russia (n= 14, 3.6%) vaccines (Figure 2A). Most of the participants received the Pfizer vaccine (n= 222, 57.4%), then Moderna and Johnson & Johnson (n= 51, 13.2%, n= 36, 9.3% respectively, and the rest received the other vaccines; Sputnik light (n= 17, 4.4%), Sinopharm (n= 3, 0.8%), AstraZeneca (n= 18, 4.7%), Covaxin (n= 33, 8.5%), as shown in (Figure 2B).
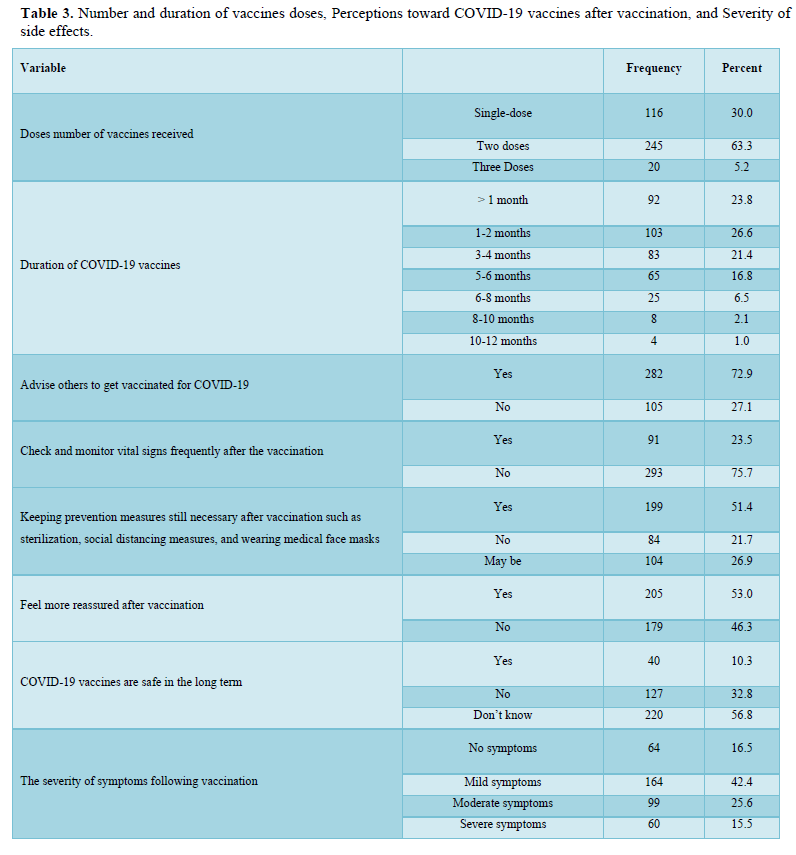
The majority of the participants (n= 245, 63.3%) have received two doses of vaccines, only 20 (5.2%) individuals received three doses and the rest of the participants (n= 116, 30%) received a single dose of the vaccination. Their duration of vaccination varied as most of them received the vaccination less than 6 months as shown in Table 3. After getting vaccinated, about half of the participants felt more reassured (n= 205, 53%) and most of them have advised others to get vaccinated too (n= 282, 72.9%). Also about half of the participants (n= 199, 51.4%) believed that keeping prevention measures such as sterilization, social distance measures, and wearing medical face masks is still necessary after vaccination, while only 40 (10.3%) of them believed that COVID-19 vaccines are safe for the long term as more than half of them (n= 220, 56.8%) didn’t know if it is safe or not. The majority of the respondents (n= 293, 75.7%) didn't monitor their vital signs frequently such as blood pressure, breathing, pulse, temperature, etc. after getting vaccinated. The majority of the participants reported they had mild (n= 164, 42.4%) to moderate (n= 99, 25.6%) side effects after vaccination. Only 60 respondents (15.5%) suffered from severe side effects and 64 (16.5%) didn’t suffer from any symptoms at all (Table 3).
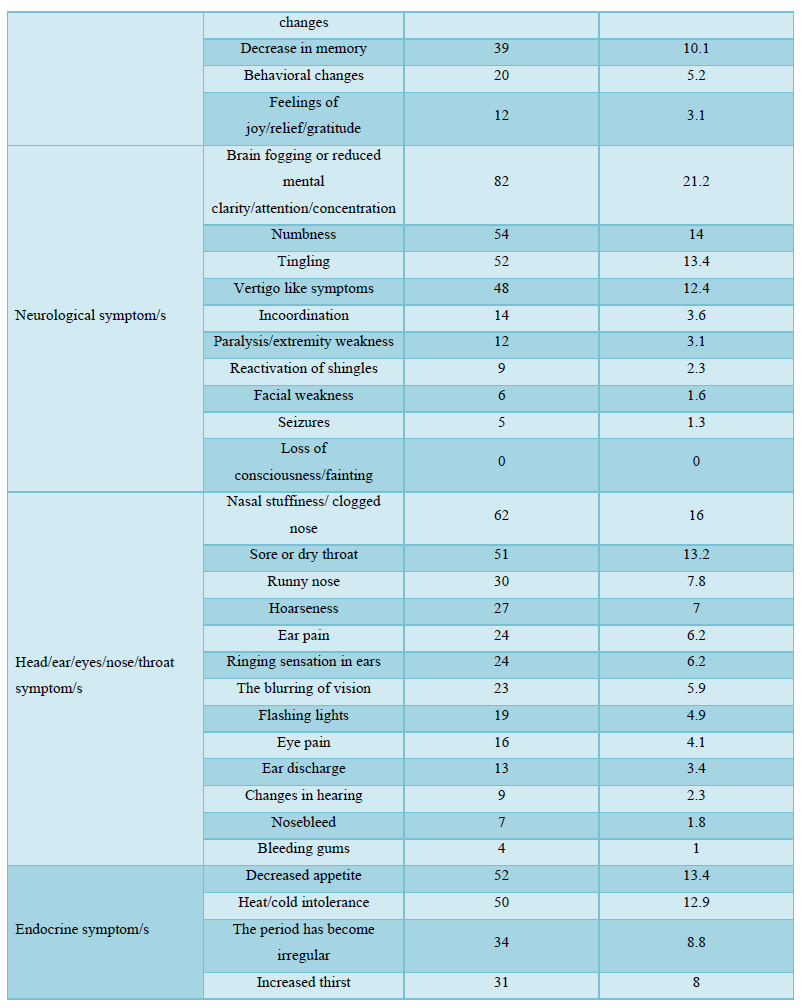
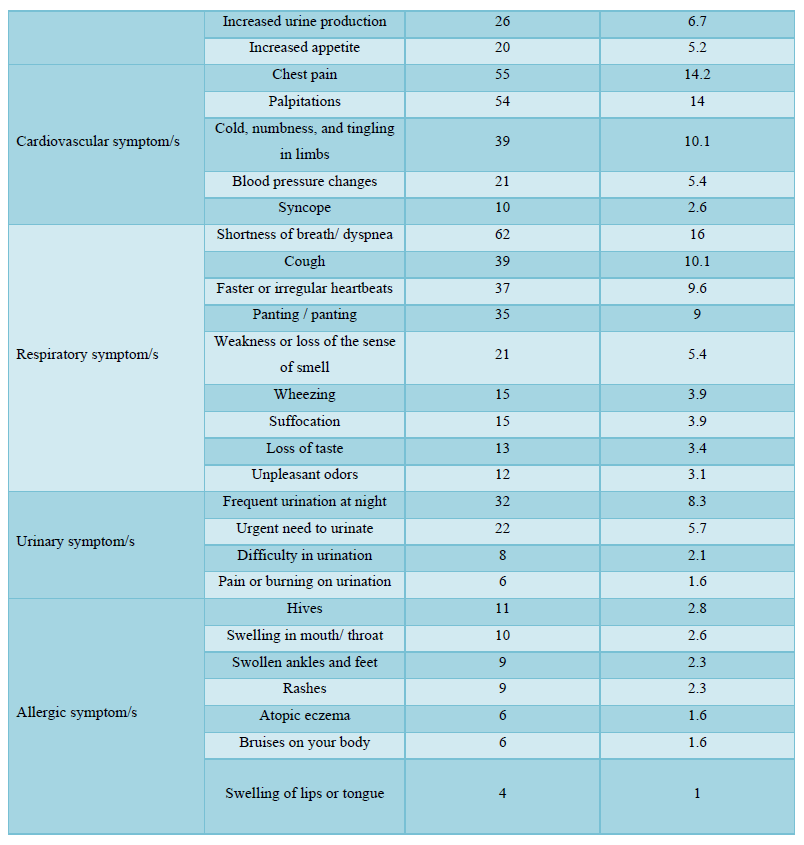
The participants in this study who received COVID-19 vaccines reported various symptoms as shown in Table 4; regardless of the type of vaccine they received, the reported symptoms are based on the organ systems. Furthermore, the relationships between several side effects of vaccination received and the different participants' variables and the number of symptoms that appeared were determined. Regardless of the types of COVID-19 vaccine received, the chi-square test analysis was used to assess the potential association between the COVID-19 vaccines' side effects and the different participants' variables.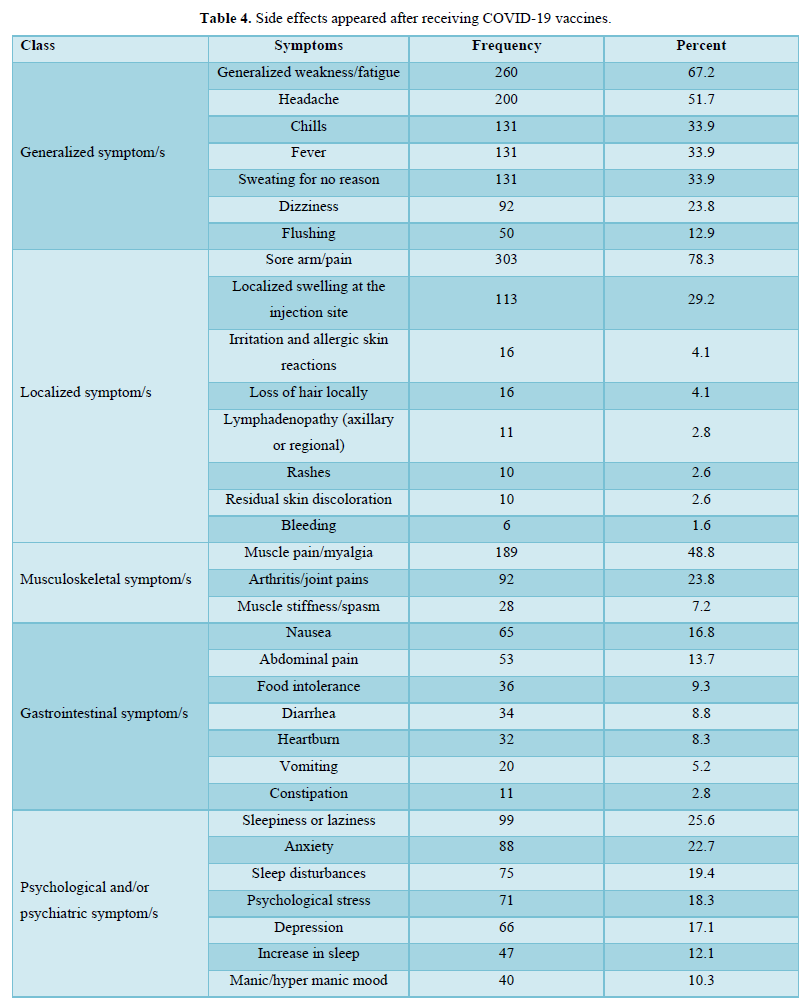
DISCUSSION
Concerns about the safety and side effects of COVID-19 vaccines have been raised among many communities worldwide including Palestine [10-13]. This study aimed to analyze the safety and potential adverse effects of COVID-19 vaccination among the Palestinian population as well as the factors associated with the side effects that appeared after vaccination. Based on the above results, the majority of the participants were from the Hebron governorate, because the questionnaire was distributed online via social media including Facebook, and the Facebook policy usually suggests the residents who are near your area. For the same reasons, most of the participants were females and young (their ages less than 30 years old). Most of the participants also with A+ and O+ blood groups, were non-smokers and didn’t suffer from any chronic disease or an allergy to any types of foods or medicines. The most common chronic diseases of the participants who have chronic diseases were diabetes mellitus, obesity, hypertension, cardiovascular diseases, joint inflammations, respiratory diseases, autoimmune diseases, thyroid disorders, dyslipidemia, and osteoporosis. The majority of the participants also is educated and have bachelor's degree, lives in cities, are married, are students, and are with low-to-middle income. Most of the participants were indeed scared to receive the vaccine, but there was high acceptance of receiving the vaccine among them, and they also indicated that it is important to get a vaccine to protect themselves and others from COVID-19, even more than half of them weren't infected with COVID-19 viruses. Their sources of information about COVID-19 vaccines mainly were from social media and government-owned media platforms. The majority of participants preferred to receive the Pfizer-Biotech-USA vaccine before vaccination, while no one preferred to get the Sinopharm-China vaccine, and few of them preferred to get the AstraZeneca/Oxford-UK vaccine; this might be due to published side effects about this type of vaccine. However, most of them received the Pfizer and then Moderna vaccines, therefore, the most abundant side effects were reported by participants who received the Pfizer-BioNTech vaccine.
The majority of the participants received two doses of vaccines and their duration of vaccination was varied as most of them received the vaccination less than 6 months. After getting vaccinated, about half of the participants felt more reassured and most of them have advised others to get vaccinated too. About half of the participants believed that keeping prevention measures such as sterilization, social distancing measures, and wearing medical face masks is still necessary after vaccination, while only a few of them believed that COVID-19 vaccines are safe for the long term as more than half of them didn’t know if it is safe or not. The majority of the respondents didn't monitor their vital signs frequently such as blood pressure, breathing, pulse, temperature, etc. after getting vaccinated. In general, the majority of the participants reported they had mild to moderate side effects after vaccination. Only a few respondents either suffered from severe side effects or didn’t suffer from any symptoms at all. The highest and the most common symptoms among the participants were sore arm/pain then generalized weakness/fatigue, headache, and muscle pain/myalgia, and no single participant lost consciousness/fainted. The other adverse effects (other than those mentioned in Table 4) that some respondents reported were feeling bloated, severe pain with a back spasm, lower extremities and severe bone pain, pain in the back, lack of focus, the appearance of pimples especially in the face, pricks, enlargement of the heart muscle and pressure in the bones of the rib cage, breathing stopped for a moment, short term memory loss, irregularity in the menstrual cycle, weakness in the extremities and tremors in the body. However, most of these appeared in vaccine recipients were not serious, they are mild to moderate as mentioned previously. The causes of the side effects or reactions to the vaccines remain unclear, but it was suggested it might be due to anaphylaxis which occurs soon after vaccination. Moreover, in our study, we identified several factors associated with vaccine adverse effects.
The comparisons between participants’ characteristics and the adverse effects of COVID-19 vaccines were also determined. It was found that all symptoms were significantly associated with gender except the respiratory and urinary, and allergic symptoms. Only generalized and psychological and/or psychiatric symptoms have a significant association with age. Smoking has a significant association with generalized and head/ear/eyes/nose/throat symptoms. It was found that there aren’t significant associations between residential location and/or blood groups and the side effects of COVID-19 vaccines received for all symptoms and there was a significant association between occupation and cardiovascular symptom/s. They were a link between food or medicine allergies and localized, musculoskeletal, psychological and/or psychiatric, neurological, head/ear/eyes/nose/throat, cardiovascular, and respiratory symptoms. There was a significant association between being infected with COVID-19 and the side effects of the COVID-19 vaccines' including musculoskeletal and cardiovascular symptoms. The type of vaccine received has a significant association with generalized and musculoskeletal symptoms (Biotech-USA vaccine). Other variables were also studied to determine if there are any relationships with the number of doses received, specific chronic diseases in the respondents (e.g. diabetes mellitus, hypertension, cardiovascular diseases, chronic respiratory diseases, obesity, joint inflammations, and thyroid disorders), and the symptoms. It was found that there were no significant associations between the number of doses received and the side effects of COVID-19 vaccines for all symptoms except for musculoskeletal symptoms, while it was a significant association between diabetes mellitus and generalized and urinary symptoms. Hypertension isn’t associated with any symptoms for the vaccines received and cardiovascular diseases are significantly associated with head/ear/eyes/nose/throat and urinary symptoms. There were statistically significant associations between chronic respiratory diseases and the side effects of COVID-19 vaccines for urinary symptoms and allergies. It was also found there aren’t significant associations between obesity and the side effects of COVID-19 vaccines. Joint inflammations have a significant association with psychological and/or psychiatric, respiratory, and urinary symptoms. Thyroid disorders and the side effects of COVID-19 vaccines have a significant association with all symptoms, except for psychological and/or psychiatric, neurological, and allergic symptoms. No significant associations were found between gender, smoking, residential location, occupation, blood type, type of vaccine, individuals suffering from diabetes mellitus, hypertension, obesity, joint inflammations, thyroid disorders, and both blood clots and thrombocytopenia and the side effects. There was also a significant association between age, suffering from food or medicines allergies, the number of doses, and thrombocytopenia and the side effects of COVID-19 vaccines. It was also found there was a significant association between cardiovascular diseases and blood clots, not thrombocytopenia. In addition, the significant associations between the vaccination side effects and chronic diseases, and the number of symptoms were also determined.
There are several techniques used for viral vaccine development, including live attenuated, inactivated, DNA-based, RNA-based, protein-based, and viral vector-based. Each technique has a specific action in the body which gives advantages and disadvantages concerning immunogenicity, safety, ease of use, and effectiveness [14]. While both Pfizer-BioNTech and AstraZeneca vaccines are developed using DNA- and RNA-based viral vectors, the Sinopharm vaccine has been developed using an inactivated virus [15]. Furthermore, the Pfizer-BioNTech vaccine was the most commonly received by participants and it also was the most preferred vaccine for participants before vaccination. This may be because it was the first vaccine approved around the world and also may be because of the huge online resources that give information about its safety and effectiveness to build immunity. More than half of the participants were scared of receiving a vaccination, this may be because of little information and clinical trials about vaccinations. However, the picture changed after vaccination; as the majority of the participants felt more reassured and advised others to get vaccinated.
Our study showed that several side effects have been reported after receiving COVID-19 vaccination, but they are not severe, most of the side effects are mild to moderate, and there was no hospitalization admission; the results of our study will reduce vaccine hesitancy take and increase its acceptance. Our results were also compatible with other studies in many countries, that COVID-19 vaccines are safe, and getting vaccinated makes people more reassured, and their side effects are mild to moderate [10,11,14-20]. To the best of our knowledge, this investigation is the first to determine the safety, potential side effects, and perceptions following COVID-19 vaccination in Palestine. There are several studies carried out in Palestine that investigated SARS-COV-2 vaccine uptake and COVID-19 vaccine hesitancy and acceptance among Palestinian healthcare workers or the general population [21-25], but there is no study investigated the safety and potential adverse effects of COVID-19 vaccination as well as the factors associated with the side effects that appeared during and after vaccination among the Palestinian population.
One of the obstacles of the study is the distributing the questionnaire online and through social media, as there is a possibility that the questionnaire will not reach all people due to the lack of internet access/people having no internet access or otherwise, thus many people are unable to participate in the study. The largest percentage of those who filled out the questionnaire is young and the percentage of the elderly is small. This may be due to the inability to reach the questionnaire through the Internet, so it is best to develop technology that helps them. Finally, the recall bias and language level of complexity could not be eliminated completely due to limitations in such studies as it is an online survey and not a face-to-face study.
CONCLUSION
Side effects of authorized COVID-19 vaccines are not serious (mild to moderate). This confirmed that they are safe and getting vaccinated makes people more reassured. The study also identified several factors associated with vaccine adverse effects.
ETHICAL CONSIDERATION
This research study was approved by the Institutional Review Board (IRB) of Arab American University. A consent statement was included about voluntary participation and anonymity at the beginning of the questionnaire.
CONFLICTS OF INTEREST
The authors have no competing interests to declare.
ACKNOWLEDGMENT
We would like to thank Safia Jbr, Salameh Ghayad, Sajeda Zeedat, Anwar Abu Snineh, and Shoruoq Khaled, who helped with the data collection.
- Kadali RAK, Janagama R, Peruru S, Malayala SV (2021) Side effects of BNT162b2 mRNA COVID-19 vaccine: A randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. Int J Infect Dis 106: 376-381.
- Hejaz HA (2020) Treatments and management of coronavirus disease 2019 (Covid-19). Int J Med Sci Diag Res 4(7): 52-71.
- Hejaz HA, Zalloum A, Shalaldeh B, Alnatsheh T, Attili R (2020) Novel Coronavirus Disease-2019: Epidemiology, Diagnosis, Therapeutics and Guideline Protocol for Disease Management in Different Countries. Iraq Med J 4(2): 2521-8492.
- Hejaz HA, Nawajah I, Wredat M, Melhem W (2022) Knowledge, attitude, and practice toward the novel coronavirus-2019 (COVID-19) outbreak: A cross-sectional study in Palestine. Adv Biomed Health Sci 1(3): 162-174.
- Hatmal MM, Al-Hatamleh MAI, Olaimat AN, Hatmal M, Alhaj-Qasem DM, et al. (2021) Side Effects and Perceptions Following COVID-19 Vaccination in Jordan: A Randomized, Cross-Sectional Study Implementing Machine Learning for Predicting Severity of Side Effects. Vaccines (Basel) 9(6): 556.
- Paul A, Sikdar D, Mahanta J, Ghosh, S, Jabed MA, et al. (2021) Peoples’ understanding, acceptance, and perceived challenges of vaccination against COVID-19: A cross-sectional study in Bangladesh. PloS One 16(8): e0256493.
- Hejaz HA, Arqoup DA, Znaid HA, Swaity H, Rabai T, et al. (2020) A review on coronavirus disease (COVID-19). Hebron Univ Res J 9(1): 45-87.
- Hejaz HA (2020) Palestinian strategies, guidelines, and challenges in the treatment and management of coronavirus disease-2019 (COVID-19). Avicenna J Med 10: 135-62.
- Hejaz HA, Fallah R, Al-Jabari R, Abdeen D, Jabari M (2021) Knowledge, Attitudes, and Acceptance toward Coronavirus Disease 2019 Vaccine. Sch Acad J Pharm 10(12): 213-224.
- Kashte S, Gulbake A, El-Amin III SF, Gupta A (2021) COVID-19 vaccines: Rapid development, implications, challenges, and future prospects. Human Cell 34(3): 1-23.
- Orebi HA, Emara HE, Alhindi AA, Shahin MR, Hegazy AH, et al. (2022) Perceptions and experiences of COVID-19 vaccines’ side effects among healthcare workers at an Egyptian University Hospital: A cross-sectional study. Trop Med Health 50(1): 37.
- Omeish H, Najadat A, Al-Azzam S, Tarabin N, Hameed AA, et al. (2022) Reported COVID-19 vaccines side effects among Jordanian population: A cross sectional study. Hum Vaccin Immunother 18(1): 1981086.
- Hernández AF, Calina D, Poulas K, Docea AO, Tsatsakis AM, et al. (2021) Safety of COVID-19 vaccines administered in the EU: Should we be concerned? Toxicol Rep 8: 871-879.
- Walach H, Klement RJ, Aukema W (2021) The Safety of COVID-19 Vaccinations-We Should Rethink the Policy. Vaccines 9: 693.
- Massoud F, Ahmad SF, Hassan AM, Alexander KJ, Al-Hashel J, et al. (2021) Safety and tolerability of the novel 2019 coronavirus disease (COVID-19) vaccines among people with epilepsy (PwE): A cross-sectional study. Seizure 92: 2-9.
- Meo SA, Bukhari IA, Akram J, Meo AS, Klonoff DC (2021) COVID-19 vaccines: Comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur Rev Med Pharmacol Sci 25(3): 1663-1669.
- Azimi M, Dehzad WM, Atiq MA, Asady BA (2021) Adverse Effects of the COVID-19 Vaccine Reported by Lecturers and Staff of Kabul University of Medical Sciences, Kabul, Afghanistan. Infect Drug Resist 14: 4077-4083.
- Babaee E, Amirkafi A, Tehrani-Banihashemi A, Azar NS, Eshrati B, et al. (2022) Adverse effects following COVID-19 vaccination in Iran. BMC Infect Dis 22(1): 476.
- Ganesan S, Al Ketbi LMB, Al Kaabi N, Al Mansoori M, Al Maskari NN, et al. (2022) Vaccine Side Effects Following COVID-19 Vaccination Among the Residents of the UAE-An Observational Study. Front Public Health 10: 876336.
- Saeed BQ, Al-Shahrabi R, Alhaj SS, Alkokhardi ZM, Adrees AO (2021) Side effects and perceptions following Sinopharm COVID-19 vaccination. Int J Infect Dis 111: 219-226.
- Alya WA, Maraqa B, Nazzal Z, Odeh M, Makhalfa R, et al. (2022) COVID-19 vaccine uptake and its associated factors among Palestinian healthcare workers: Expectations beaten by reality. Vaccine 40(26): 3713-3719.
- Maraqa B, Nazzal Z, Rabi R, Sarhan N, Al-Shakhra K, et al. (2021) COVID-19 vaccine hesitancy among health care workers in Palestine: A call for action. Prevent Med 149: 106618.
- Rabi R, Maraqa B, Nazzal Z, Zink T (2021) Factors affecting nurses' intention to accept the COVID-19 vaccine: A cross-sectional study. Public Health Nurs 38: 781-788.
- Zawahrah HJ, Saca-Hazboun H, Melhem SS, Adwan R, Sabateen A, et al. (2021) Acceptance of COVID-19 vaccines in Palestine: A cross-sectional online study BMJ Open 11(10): e053681.
- Qasrawi H, Abdullah I, Masri H, Maraqa B, Mohammad A, et al. (2022) Perceived barriers to Palestinian pregnant women’s acceptance of COVID-19 vaccination using the Health Believe Model: A cross-sectional study, Women Health 62(8): 678-687.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Allergy Research (ISSN:2642-326X)
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)
- Journal of Infectious Diseases and Research (ISSN: 2688-6537)
- International Journal of Internal Medicine and Geriatrics (ISSN: 2689-7687)
- Chemotherapy Research Journal (ISSN:2642-0236)
- Journal of Neurosurgery Imaging and Techniques (ISSN:2473-1943)
- Journal of Rheumatology Research (ISSN:2641-6999)



