Case Report
HHV- 6 Infections in Immunocompetent with Multiorgan Failure
4590
Views & Citations3590
Likes & Shares
We report a case of 32-year-old male patient previously fit with no co morbidities, presenting initially as acute undifferentiated febrile illness that on investigation was found to have human herpes virus 6 infection of the central nervous system. There is various case report of HHV 6 infection of CNS in an immunocompetent individual reported in literature but our case is different as it presented with associated MODS and not just with CNS involvement. We herewith report our case and the workup and the treatment which led to our conclusion of HHV6 infection in a young immunocompetent male with multiorgan involvement at presentation.
INTRODUCTION
Human B - lymph tropic virus was first isolated in 1986 from the peripheral blood mononuclear cells of a persons with HIV infection or lymph proliferative disorders, [1] and the virus was designated “human herpesvirus 6 (HHV -6). It belongs to DS DNA virus part of the Herpes viridae family [2].
HHV 6 found to cause rosella infantum it has been categorized into variants A and B [1]. Evidence suggests that HHV-6B primarily appears in children with rosella or other febrile illnesses. HHV-6A is primarily found in patients with lymph proliferative disorders and in immunocompromised hosts with CNS disease [1].
Human herpesvirus type 6 (HHV-6) and cytomegalovirus (CMV) are known to interact with the production of cytokines. In this study, we sought to determine the incidence of HHV-6 and CMV reactivation during multiple organ failure syndrome (MOFS) and to evaluate the potential effects of viral replication on both the morbidity and mortality associated with MOFS [3].
Most individuals are infected with HHV-6 in childhood, but many are asymptomatic. Its occurrence is rare in immunocompetent patients, but there have been several reports of encephalitis due to HHV-6 reactivation [4]. We report a very rare case of an immunocompetent adult man of 32 years with encephalitis with multiorgan failure due to HHV -6.
CASE PRESENTATION
A 32-year-old male with no previous illnesses got admitted with 10 days’ h/o fever, cough with minimal expectoration, loose motion and breathing difficulty of 3 days duration. Patient was treated for the above complaints by a general practitioner prior to admission.
On admission he was febrile (temp 101.9°F), HR 102/m BP 150/70 mmHg, RR 24/min andSpO2 100% on Oxygen 8 lit/min with HGT of 102mg/dl. Relevant general physical examination revealed jaundice, congested eyes and Maculopapular rash all over body.
On systemic examination RS- B/L coarse crepitations in mid and lower zones, CVS- S1S2+, tachycardia, no added sounds, murmur or rub.PA- mild right hypochondriac and epigastria tenderness. CNS- he was conscious, mildly drowsy with a GCS of E4V4M6 and no lateralizing signs. Skin examinations suggestive of Diffuse macular and few Maculopapular lesions in the distribution of upper limbs and lower limbs, proximal > distal with malaria lesions with few purpuric eruptions on trunk and minimal conjunctival congestion. There were no evidence of vesicles or bullae with palms and soles being spared and no evidence of other mucosal involvement or deep dermal tenderness with absent nikolsky’s sign.
Patient did not have history of contact with any patient with fever in the current COVID pandemic. Patient also denied history of any travel in preceding 6 weeks. Personal history, non-alcoholic, non-smoker. Family history was non-contributory. Patient clinically deteriorated in the next 48 h developed status epileptics and decreased urine output requiring renal replacement therapy. Patient had to be intubated in view of GCS < 8 post convulsions.
INVESTIGATIONS
Patient was initially worked up as an acute undifferentiated febrile illness. Multiplex fever panel was negative for malaria, dengue, chikungunya, leptospirosis, scrub typhus and typhoid. Laboratory results revealed by cytopenia with hemoglobin dropping from 14.8gm% to subsequent less than 8gm% with serial rise in WBC counts from 8900/mm3 to 22,260/mm3. Platelet counts dropped from 193,000/mm3 to less than 20000/mm3 in span of 7 days. Other system relevant investigations on presentation revealed BUN/creatinine of 79.1/9.8mg/dl, bilirubin of 4.2 mg% (direct 3.37), alkaline phosphatase 1237 with mildly deranged aminotransferases (SGPT/SGOT 66mg/dl/54mg/dl), Na 119, K- 5.9, Cl- 86.5, coagulation profile normal.
Radio diagnostics imaging done Abdominal CT showed hepatosplenomegaly, abdominal Lymphadenopathy, mild ascites, medical renal diseases, normal pancreas.
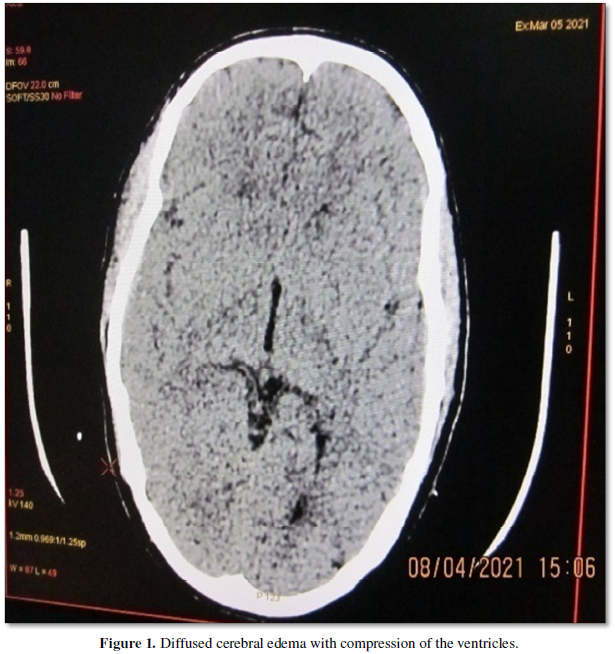
CT Brain showed diffuse cerebral edema with compression of the ventricles (Figure 1).

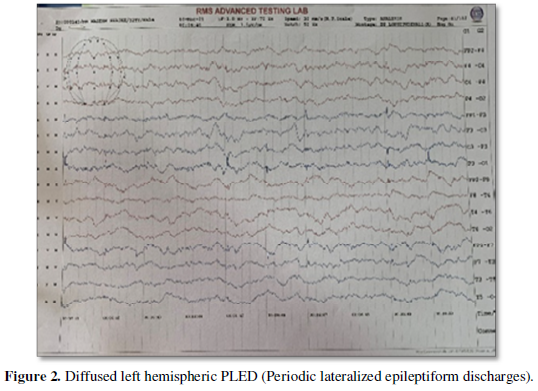
EEG showed diffuse left hemispheric PLED (Periodic lateralized epileptiform discharges) (Figure 2).

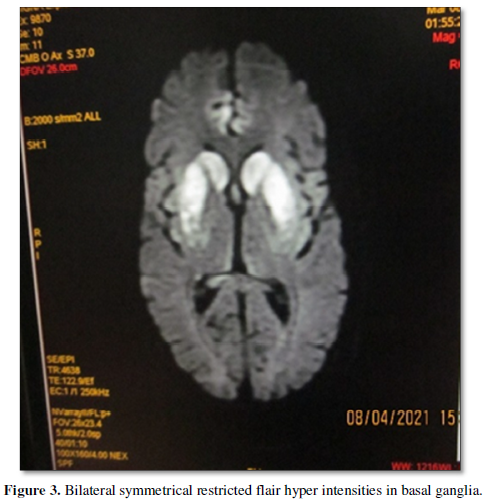
MRI brain done after treatment of cerebral edema a week later after CT brain revealed bilateral symmetrical restricted flair hyper intensities in basal ganglia, medial temporal lobe suggestive of viral encephalitis (Figure 3).

CSF analysis post MRI brain imaging- Protein 144, sugar 56.4, chloride 125, 15 cells- 64% polymorphs, 20% lymphocytes, 16% monocytes. Gene Expert negative. CSF bio fire revealed HHV 6. Bone marrow aspiration with biopsy and culture revealed cellular reactive bone marrow with trilineage hematopoiesis and culture no growth after 5days of incubation at 37°C.
TREATMENT
Initially patient was put on antibiotics- Piperacillin/tazobactum, Clarithromycin, antimalarials-artemisinin derivatives (falcigo) and supportive care. Antibiotics were upgraded in view of worsening clinical situation to meropenem and teicoplanin. Eventually CSF bio fire testing detected HHV6 DNA in the CSF and the patient was started on Ganciclovir. Respiratory care bundles and other critical care bundles were adhered to in the management.
DISCUSSION
Our case represents a severe form of HHV6 infection with multiorgan dysfunction syndrome. A review of 31 cases of HHV6 CNS involvement in immunocompetent adult with varied presentation has been described in literature. HHV - 6 associated with immunosuppressive and organ transplanted patients are well established [5].
Isakson [1] found that HHV6 causes encephalitis in 4 immunocompetent patients. Traubel [4] have been reported HHV6 associated meningoencephalitis in an immunocompetent adult. A cause still elusive to many, probable reason for neuron-invasion and reactivation plays a role.
However very few case reports have reported multisystem involvement. Desachy [3] stated that multiorgan failure syndrome appeared due to reactivation of HHV-6 in immunocompetent adults. Wang [2] reported a case of HHV6 infection in an immunocompetent patient with multiorgan failure. Our patient had encephalitis with kidney injury and liver involvement.
There is no standardized work up and no definite treatment known as it presents like any acute febrile illness. In most cases the screening test is a NAAT of CSF, Blood or tissue and recommends confirmatory testing for definite diagnosis. In our case to the diagnosis was confirmed by bio fire assay in CSF. The study by Yao [6] gives hint in interpreting NAAT screening and recommends confirmatory testing after NAAT for definite diagnosis. Ganesh [7] used PCR test of CSF for the diagnosis and intravenous Ganciclovir or foscarnet is chosen for the treatment for HHV6 meningitis in an adult as a first line. Traubel [4] study used CSF PCR, neuroimaging for diagnosis of HHV6 DNA and treated successfully by Ganciclovir and valganciclovir in case of meningoencephalitis with HHV6 in immunocompetent adult.
Neurotropic viruses such as HHV6, Herpes has the ability to incorporate into the host genome remaining latent in peripheral blood, mononuclear cells, neural cells and other brain tissues after a primary infection usually in childhood. Activation of the virus from the latent state causing symptoms which can range from a febrile presentation to acute febrile undifferentiated fever such as in our case. A multicenter evaluation of bio fire film array (bio fire diagnostic (Utah US) meningitis encephalitis panel reported that HHV6 has the worst sensitivity of all pathogen tested at 85.7% and specificity of 99.7%. our bio fire analysis of the CSF picked up HHV6 in the CSF.
Treatment of HHV6 infection varies according to presenting clinical situations. In infants with rosella infantum where HHV6 is a causative organism treatment is mainly supportive [8]. Drugs such as ganciclovir or valganciclovir and forscarnet have been used. Treatment with individual with severe acute HHV-6 infection remains under investigation. Experts recommend Ganciclovir, foscarnet for the treatment and duration of therapy is varying case to case. For monitoring therapy of HHV6, it has been proposed that viral load may be used to assess disease progression and duration of therapy.
Trabue [4] suggested that clinical outcome is generally poor, with high morbidity and mortality based on the limited data available. In our case patient’s hospital course was prolonged and complicated by persistent encephalopathy, seizures and multiorgan failure. He continued to decline both cognitively and functionally, and died in April 2021.
CONCLUSION
We believe in our case report that HHV-6 infection should be considered early in the differential diagnosis of acute undifferentiated febrile illness and early recognition may help timely intervention to prevent both morbidity and mortality which has been reported in severe HHV6 infection.
However, the clinical role of HHV-6 infection might be underestimated, as HHV-6 diagnostics is not routinely performed. Fever, myelosuppression, and end‐organ disease including hepatitis, gastrointestinal disease, acute kidney injury and encephalitis have been associated with HHV-6.
- Isaacson E, Glaser CA, Forghani B, Amad Z, Wallace M, et al. (2005) Evidence of human herpesvirus 6 infections in 4 immunocompetent patients with encephalitis. Clin Infect Dis 40(6): 890-893.
- Wang RN, De Oleo RR, Gupta SS, Ynoa DY, Kupfer Y (2019) HHV-6 Infection in an Immunocompetent Patient with Multi-Organ Failure. Cureus 11(8): 10-12.
- Desachy A, Ranger-Rogez S, Francois B, Venot C, Traccard I, et al. (2001) Reactivation of Human Herpesvirus Type 6 in Multiple Organ Failure Syndrome. Clin Infect Dis 32(2): 197-203.
- Trabue CH, Bloch KC, Myers JW, Moorman JP (2008) Case report and literature review: HHV-6-associated meningoencephalitis in an immunocompetent adult. Herpes 15(2): 33-35.
- Lautenschlager I, Razonable RR (2012) Human herpesvirus-6 infections in kidney, liver, lung, and heart transplantation: Review. Transplant Int 25(5): 493-502.
- Schoenbergb MSFFP, MacGibbonc K, Mullind P, Romeroe R (2008) Genetic changes NIH. 23(1): 1-7.
- Maniam GB, Wilkerson H, Milton JS (2020) Human herpesvirus 6 meningitis in an adult. Baylor Univ Med Cent Proc 33(2): 246-247.
- Agut H, Bonnafous P, Gautheret-Dejean A (2015) Laboratory and clinical aspects of human herpesvirus 6 infections. Clin Microbiol Rev 28(2): 313-335.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Biochemistry and Molecular Medicine (ISSN:2641-6948)
- Journal of Genetics and Cell Biology (ISSN:2639-3360)
- Journal of Womens Health and Safety Research (ISSN:2577-1388)
- Advances in Nanomedicine and Nanotechnology Research (ISSN: 2688-5476)
- Journal of Genomic Medicine and Pharmacogenomics (ISSN:2474-4670)
- Journal of Veterinary and Marine Sciences (ISSN: 2689-7830)
- Proteomics and Bioinformatics (ISSN:2641-7561)





