Case Report
Mycotic Infections Causes Histopathological Alterations in Skin and Liver of Channa Punctatus from Amravati
5374
Views & Citations4374
Likes & Shares
Histopathology changes were observed to assess the effect of fungus on skin and liver of Channa punctatus. Histological observations of the infected fish liver showed; hemorrhage, blood congestion and necrotic cells. However, the fungal infection is more abundant in the captive environment which adversely affects fish industry. An external fungal infection cause lesions, subsequently become enlarge and may lead death. Stress, physical injury, malnutrition and poor water quality increase the susceptibility of fungal infections. The alterations found in these organs are easier to identify than functional ones and serve as warning signs of damage to animal health. Hence, the study of tissue deformities in respect to fungal infection which is commonly encountered in freshwater aquaculture needs to be studied in order to attain maximum yield. Keeping this in mind, the present study was carried out in order to understand and describe the degree of histological alterations in skin and liver of fungal infection.
Keywords: Edible fishes, Fungal infections, Histopathology, Skin, liver
INTRODUCTION
Fish is a vital source of food for people. It is man’s most important single source of high-quality protein, providing 16 % of the animal protein consumed by the world’s population, according to the Food and Agriculture Organization (FAO) of the United Nations. In many Asian countries over 50% of the protein intakes comes from fish while in Africa the proportion is 17.50% [1]. From the view point of Inland fish production, India ranks third in the world [2]. Potentially the vast and varied inland fishery resources of India are one of the richest in the world. But so far, the availability is concerned; the average animal protein available per inhabitant per day in India is 6 gm, good quality of fish for consumption can only be produced in an environment free from pathogens. Fish, having great economic importance, are affected immensely by variety of pathogenic agents in various ways. Several reports indicate high mortality of juvenile fish and reduced breeding potentiality of adults after long term microbial infections [3-5]. Large scale mortality of the fresh water fishes is often experienced due to environmental stresses followed by pathogenic attacks and parasitic infections causing a tremendous loss to the world economy.
Histopathology study of some fungal infected fishes investigated. The study showed the variation in symptoms of infections in different fish species. Gills and body surface were the main infested areas and gill filaments were damaged by the parasites. The parasites were first found to settle on the body surface and tip of the gills of fishes and attached themselves on the surface by their powerful sucking devices. The histochemical studies showed that the disease caused extensive damages to the blood elements by rupturing blood capillaries, causing necrosis, coagulation and hemorrhage [6].
In India, fishers are facing serious problems with fungal diseases. Fungi are mostly attacked due to changes in temperature and filthy conditions of water, which causes excessive zoospores to grow. Fishes not only play a vital role in the demand of food for human being but they have also emerged as main model organisms for various biomedical researches [7,8]. The ammonia which is formed by the rotting of fish waste wears is also causative factor for fungal infection [9].
MATERIALS AND METHODS
Study area
Wadali Lake is located at 20°93” N and 77075” E and at an elevation of 343m in Amravati, Maharashtra (India). It is in the vicinity of Sant Gadge Baba Amravati University campus towards South - East direction of the university in the Pohara range of hills. The lake was constructed to meet the severe water scarcity problem erupted in 1899. The lake is surrounded by open hills towards east which drain water during monsoon. The lake also receives waste water from the Wadali zoo and forest quarters from South and drainage from SRP (State Reserve Police) Camp from North. It is also watered by a percolation channel from upper Wadali Lake. When it fills to its maximum capacity the excess water overflows through an outlet, located on west shore of the lake to form Amba Nalla.
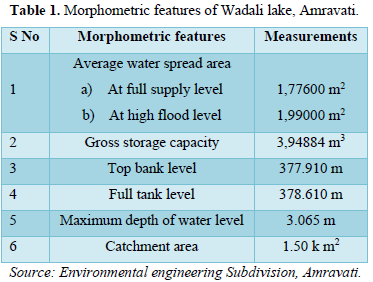
The water from the lake is being used for the drinking purpose and fishery activities, where the fishing of Channa punctatus (Phool-dhok), Catla catla (Katla), Labeo rohita (Rohu), Clarias species (Mangri), Wallago attu (Shivada), Mystus seenghala (Singala) is carried out on commercial scale. The morphometry of Wadali Lake is given in the Table 1.

Collection of fishes and Sampling techniques
The healthy and infected Channa punctatus were collected randomly every week at regular interval from the study area with the help of fishermen. The infected fishes in catch were identified from red spot on their body, excess mucus secretions, damaged and infected gills and their sluggishness. For further investigations like isolation and culture of infective Fungi to know their morphotaxonomy and pathogenicity to fishes, histopathological alterations in various organs and alterations in muscle protein contents the healthy as well as infected fishes were brought to the laboratory immediately after collection. They were acclimatized at laboratory condition in big aquaria (48x18x18) inches for 15 days.
Isolation of fungus: Following steps were followed for the fungal studies.
- Potato Dextrose Agar (PDA) media was used as a culture media for the isolation of the fungus.
- Infected fishes were cut in cross section, using a flamed scalpel.
- Small block of muscle was removed from the lesion.
- Blocks of tissue were removed and placed into Petri dishes, washed with 15 ml distilled water.
- The tissue blocks were transferred into the other set of Petri dishes.
- The Petri dishes were placed inverted in incubator at 25°C for 3 days, until a circular fungal mat developed, which were used for subculture of the fungus.
- A suitable portion of culture of different colonies from PDA was taken out with the help of forceps or needle and put on a slide in 1 or 2 drops of cotton blue on clear slides and examined under a compound microscope.
Histopathological studies
To study the histopathological alterations induced due to the fungal infections in C. panctatus the tissues namely skin and liver were dissected out from both control and infected fishes and rinsed in fresh water fish saline to remove the bloodstains and tissue debris. Then all the tissues were cut into small pieces of desirable size and fixed immediately into aqueous Bouin’s fluid. All possible precautions were taken to ensure proper fixation of tissues. The tissues were further processed by standard methods as described by Weissman [10]. The sections were cut and stained with hematoxylin-eosin, processed further, cleaned and then observed under microscope.
RESULTS AND DISCUSSION
The fishes with external lesions were found to be infected by Fungi. Among the five species selected for present study three were found to be infected by Fungi. Channapunctatus were badly infected and hence were selected for further studies. The infected fishes examined exhibited gross external and internal lesions. Fungi on fishes detected as grey or white patches on the skin and gills. The first indication of the fungal infection was the dullness of the body color, abnormality of the scales on the body surface and later on the fungus observed as a small tuft, on the body. The activities of the fishes were greatly lowered. The size of area of skin and gill damage determined the severity of the disease. In severe infected fishes were found to be degenerated more.
During study period out of 629 Channa punctatus 123 were infected (19.55%), and in case of Clarias sp. out of 593 fishes 97 were found to be infected i.e., 16.35%. The least affected fish species was Heteropneustes fossillis in which out of 189 fishes only 10 were found to be infected i.e., 5.29%. No fungal infection was recorded in Labeo rohita and Catla catla.
Infected fishes exhibited twelve different fungal infections. The fungal infected tissue was cultured on appropriate culture media and the microscopic observations showed the presence of fungal species like Achyla hypogyna, Alternaria alternata, Aphanomyces invadans, Aspergillus flavus, Aspergillus Niger, Cladosporium cladosporides, Curvularia lunata, Drechslera hawaiinsis, Fusarium oxysporum, Mucor mucedo, Rhizopus stolonifer and Saprolegnia parasitica. Clinical signs of fungal infection were more severe in the month of October, November, December and January, where as in the month of March and April most of the fishes were not infected and were healthy. The fishes with pale appearance with lesions were considered for fungal isolation studies, similar observations were made by Refai [11]. Fungal infections in freshwater fishes are common, worldwide and are associated with immune suppression [4]. Fungal infections are easily recognized by relatively superficial, colony of fluffy growth on the skin and gill of fishes. Fungal infections in fishes cause clinical abnormalities such as skin darkening, exophthalmia, corneal opacity, abdominal distention, ulceration of the skin and cotton wool like growths on various parts of the body.
To study the of impact of fungal infection on the organs like skin and liver, sections passing through the lesions of these organs of infected fishes were studied. All the infected fishes with lesions examined showed the similar pathological changes with diffused infiltration of broad, non-septate fungal hyphae and a large number of inflammatory cells through the lesion.
Histopathological alterations due to fungal infections on the skin
To know the effect of fungal infection on the histological architecture of the fish, tissue like skin and kidney were selected in both the fishes under study. All reported fungi affected skin. Saprolegnia parasitica affected kidney as well as skin and they were reported from all those tissues. Infection of A. hypogyna, A. invadens, C. cladosporides and F. oxysporum was also observed in gills. However, except S. parasitica no fungi were noted in kidney and liver. Because of the occurrence of S. parasitica in all the four tissues, it was decided to study the histopathological alterations in all the four tissues skin and kidney infectedin C. panctatus.
Structure of skin of control fishes
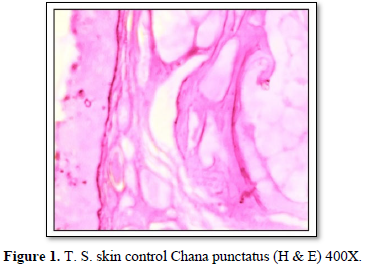
Histologically, skin of control fish showed two major cutaneous layers (epidermis and dermis) and a subcutaneous layer (hypodermis). The epidermis consisted of three types of cells, secretory cells, epithelial cells and a few taste bud’s cells. The dermis is separated from the epidermis by a basement membrane. Dermis showed fibroblasts, melanophores and blood vessels. Hypodermis is characterized by vascularized adipose tissue (Figure 1).

Histopathological alterations in skin of infected fishes
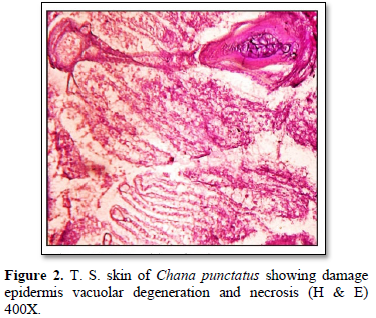
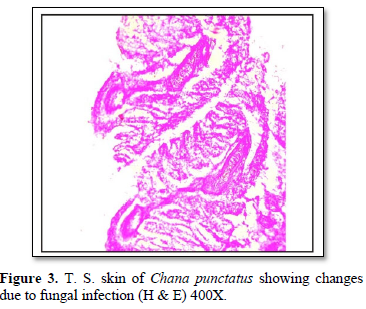
In Hematoxylin and Eosin-stained sections, infected with Saprolegnia, numerous hyphae are seen on the skin surface, beneath which are areas of degenerating tissue ranging from superficial dermal necrosis and oedema to deep myofibrillar necrosis and extensive hemorrhage. After severe skin infection of S. parasitica, the skin exhibited showed vacuolation, shrinkage, hyaline degeneration, separation of muscle fibers from each other and damage to muscle fibers leading to degeneration and necrosis (Figures 2 & 3). Fungal hyphae were also observed in the section particularly in sub dermal muscle layer with severe floccular degeneration of the muscle. Granulomas were formed around the penetrating hyphae.


Histopathological alterations in liver
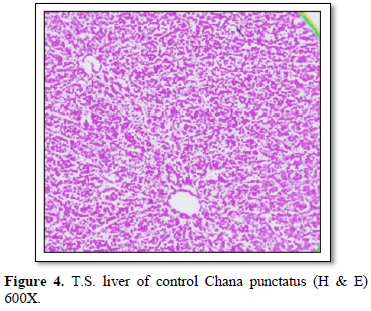
- S. of healthy liver shows continuous mass of hepatic parenchymal cells arranged in cords around central blood vessels. The hepatic cells are polygonal in shape with centrally placed rounded nucleus and homogeneous cytoplasm. Hepatic cells are not arranged to form distinct lobules. Pancreatic acini of exocrine function lie embedded in between the hepatic cells surrounding the blood capillaries. The sinusoids irregularly distributed between the hepatocytes. The biliary system occurs as intercellular bile canaliculi. The rows of hepatic cells are supported by a thin layer of connective tissue (Figures 4-6).
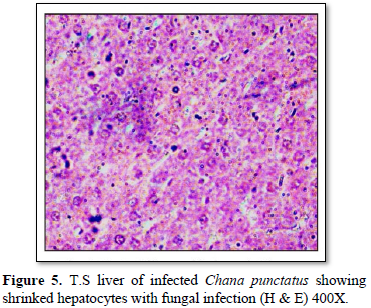
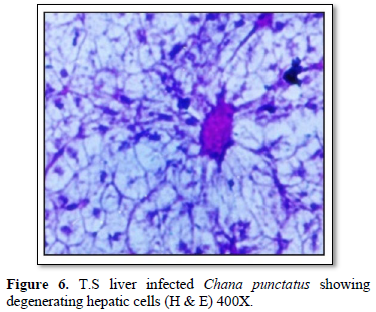
Histological observation of liver of the infected fishes generally showed; hemorrhage, blood congestion and necrotic cells. The hepatocytes were severely affected showing irregular-shaped shrinked nuclei (Figure 5). In infected tissue focal infiltration leading to fungal granuloma, with focal necrosis was observed. Hepatocytes were lost in many places showing empty spaces in liver and blood cells were observed with severe necrotic regions (Figure 6).
Histopathological studies of lesions in various tissues such as skin and liver of all the infected fishes showed diffuse infiltration of broad, non-septate fungal hyphae and a large number of inflammatory cells through the lesion. However, fungal parasitic infections were more in the skin and muscles (Figures 2 & 3) [12,13] also recorded similar findings and are of the opinion that distinctive inflammatory response may be produced against the fungus by the immune defenses of the fish. Srivastava and Srivastava [14] identified fungal infection in Channa punctata and opined that the infection started due to netting injury and over-crowding. In an attempt to halt the invasion of fungus through the tissues of the fish, macrophages surround and envelop invading mycelium by forming granulomas [15]. Severely infected fishes showed necrosis of skin in dorsoventral and caudal region with hemorrhagic lesions (Figures 2 & 3). Eli [16] recorded similar findings while reviewing the fungal infection in African fishes. Deep necrosis turned into infection and extended up to tail region in the diseased fishes.
The texture of skin of infected fishes was totally changed and showed aberrant conditions. Histologically, vacuolation, shrinkage and degeneration and necrosis of muscle were observed (Figures 2-4). These findings are similar with Zahura [17]. Chronic necrosis of muscle tissue with numerous mycotic granulomas was found frequently in the affected muscle tissue. The positive diagnosis of this was made by detecting the presence of mycotic granulomas in affected fish tissues through histological findings which is correlated with the observations of EUS affected freshwater fishes of South and Southeast Asia [18]. Mohan and Shankar [19] also described numerous granuloma formations as results of chronic inflammatory response to fungal hyphae in EUS affected fishes. Similar mycotic granulomatosis was also reported from fishes in Japan [20,21].
All the examined fishes showed diffused infiltration of broad, non-septate fungal hyphae and a large number of inflammatory cells through the lesion in Saprolegnia infected fishes. Very few hyphae penetrated the muscle fibers but, no inflammatory response was found around them and most of the muscle fibers were degenerated but few enveloped fungal hyphae were also observed (Figure 2). Similar findings have been recorded by Roberts [12,22].
The presences of massive numbers of highly invasive, broad non-septate Fungi were recorded by Pathiratne and Jayasinghe [23] in EUS affected fishes. Lilley [24,25] reported that a specific fungal pathogen, A. invadans, is the necessary cause of EUS.
Kiryu [26] while working on Atlantic menhaden, Brevoortia tyrannus, reported that cyst stages were infectious because they attached to the intact skin of and produced germination tubes that penetrated the skin. China but Kiryu [26] reported that distinctive inflammatory response may be produced against the fungus by the immune defenses of the fish to halt the invasion of fungus through the tissues of the fish; macrophages surround and envelop invading mycelium by forming granulomas. A decrease in mucus cells and presumptively mucus production has been proposed to increase risk of fungal infection [27].
Histological observations of the infected fish liver showed; hemorrhage, blood congestion and necrotic cells. The hepatocytes were severely affected showing irregular-shaped shrinked nuclei (Figure 5). In infected tissue focal infiltration leading to fungal granuloma, with focal necrosis was observed (Figure 2). Hepatocytes were lost in many places showing empty spaces in liver and blood cells were observed with severe necrotic regions (Figure 6). Similar findings are observed by Chandra [28], Camargo and Martinez [29].



CONCLUSION
Although most of the fishes from the lake were found to be healthy, but under histopathological observations it was found that most of fishes were affected by fungal parasites. Histopathological studies of lesions in various tissues such as skin, gill, liver and kidney of all the infected fishes showed diffuse infiltration of broad, non-septate fungal hyphae and a large number of inflammatory cells through the lesion in Saprolegnia infected fishes. However, fungal parasitic infections were more in the skin and muscles. Severely infected fishes showed necrosis of skin in dorsoventral and caudal region with hemorrhagic lesions. Histological observations of the infected fish liver showed; hemorrhage, blood congestion and necrotic cells. The hepatocytes were severely affected showing irregular-shaped shrink nuclei. In infected tissue focal infiltration leading to fungal granuloma, with focal necrosis was observed. Histopathological kidney of infected fishes showed vacuolation, hemorrhage, mild tubular degeneration with fungal granuloma, hematopoietic tissue degeneration and cloudy swelling to uriniferous tubules with glomerular expansion. Inflammatory lymphocytes were also observed in the intratubular spaces. Gill lamellae of infected fishes showed hypertrophy and hyperplasia. The tips of secondary lamellae of gills exhibited peculiar malformations such as curving as well as cellular hyperplasia and clubbing of tips. Destruction and ulceration of the gill lamellae leading to perforation of the basement membrane and the capillaries ultimately resulted in the gill hemorrhage. Degeneration of the infected gills affected respiration and eventually the fish died of suffocation. Most fish died due to osmotic or respiratory problems if the affected area of skin or gills was large. From the survey and histopathological findings, it is revealed that Channa punctatus and Clarias sp. were the most severely affected species. Management point of view, it is needed to extend proper attention towards C. punctatus and Clarias sp. Moreover, from the present studies, it can be suggested that more precautionary measures have to be undertaken at the onset of winter season, so that incidences of fungal infections in fishes can be avoid [30].
- William CF, Dennis CW (1988) Food Microbiology, 4th edn, food science series. McGraw-Hill Book Company, Singapore, pp: 243-252.
- World Bank (2010) India marine fisheries, issue opportunities and transition for sustainable developments Agriculture and rural development sector unit south Asia region. Report No. 54259.
- Nicola F (1969) Systemic mycosis in channel catfish. Wildl Dis 5: 109-110.
- Pickering AD, Willoughby LG (1982a) Saprolegnia, infections of Salmonid fish. In: 50th Annual Report, Institutes of freshwater Ecology, Windermere Laboratory, England, pp: 38-48.
- Hatai K, Hoshiai G (1992) Mass mortality in cultured Coho salmon (Oncorhynchus kisutch) due to Saprolegnia. Parasitica Coker. J Wildl Dis 28: 532-535.
- Hossain K, Hossain DM, Rahman HM (2007) Histopathology of some diseased Fishes. J Life Earth Sci 2(2): 47-50.
- Patel AS, Patel SJ, Bariya AR, Pata BA, Ghodasara SN (2018) Fungal diseases of fish: A review. J Vet Sci Res 3(3): 000164.
- Rao PK (2020) Fungal Infection Causes Histopathological Alterations in Gill and Pancreas of Channastriatus, Int J Pharm Sci Res 11(4): 1784-1789.
- Chauhan R, Bhatt MH, Lone SA (2014) Pathogenic Effects of Three species of fungi. (Aphanomyces laevis, Aspergillus Niger and Saprolegnia parasitica) on Gold Fish (Carassius auratus L). Indo Global J Pharm Sci 4(2): 41-46.
- Weissman K (1972) In General, Zoological Micro techniques, Blakiston Company. Philadelphia.
- Refai MK, Mohamed LA, Kenawy AM, Shimaa El-SMA (2010) The assessment of mycotic settlement of freshwater fishes in Egypt. J Am Sci 6(11): 595-602.
- Roberts RJ, Campbell B, MacRae IH (1994) Proceedings of the ODA regional seminar on Epizootic Ulcerative Syndrome. Aquatic Animal Health Research Institute, Bangkok. pp: 282.
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25: 4876-4882.
- Srivastava GC, Srivastava RC (1977) Host range of AchlyaproliferaNees De Bary on certain fresh water teleosts. Mycopathologia 61(1): 61-62.
- Chinabut S, Roberts RJ, Willoughby GR, Pearson MD (1995) Histopathology of snakehead, Channastriatus (Bloch), experimentally infected with the specific Aphanomyces fungus associated with Epizootic Ulcerative syndrome (EUS) at different temperatures. J Fish Dis 18: 41-47.
- Eli A, Briyai OF, Abowei JFN (2011) A Review of Some Fungi Infection in African Fish Saprolegniasis, Dermal Mycoses; Branchiomyces infections, Systemic Mycoses and Dermocystidium. Asian J Med Sci 3(5): 198-205.
- Zahura UA, Chowdhury MBR, Faruk MAR (2004) Fungal infection in the freshwater fishes of Mymensingh area in Bangladesh. Indian J Fish 51: 61-68.
- Roberts RJ, Willoughby LG, Chinabut S (1993) Mycotic aspects of Epizootic Ulcerative Syndrome (EUS) of Asian fishes. J Fish Dis 16: 169-183.
- Mohan CV, Shankar KM (1995) Role of fungus in Epizootic Ulcerative Syndrome of fresh- and brackish water fishes of India: A histopathological assessment. In: Shariff, M.; Arthur, J. R. and Subasinghe, R. P. (Eds), Diseases in Asian Aquaculture II. Fish Health Section, Asian Fisheries Society, Manila. pp: 299-305.
- Miyazaki T, Egusa S (1973) Studies on mycotic granulomatosis in freshwater fishes-IV. Mycotic granulomatosis in wild fishes. Fish Pathol 8: 44-47.
- Hatai K (1980) Studies on pathogenic agents of Saprolegniosis in freshwater fishes Special Report on Nagasaki Prefectural Institute of Fisheries 7: 95.
- Vishwanath TS, Mohan CV, Shankar KM (1997) Clinical and histopathological characterization of different types of lesions associated with Epizootic Ulcerative Syndrome I. Aquaculture Imp 12: 36-42.
- Pathiratne A, Jayasinghe RPPK (2001) Environmental influence on the occurrence of Epizootic Ulcerative Syndrome (EUS) in freshwater fish in the Bellanwila - attidiya wetlands Sri Lanka. J Appl Ichthyol 17(1): 30-34.
- Lilley JH, Callinan RB, Chinabut S, Kanchanakhan S, MacRae IH, et al. (1998) Epizootic Ulcerative Syndrome (EUS) technical handbook. Aquatic Animal Health Research Institute and Network of Aquaculture Centers in Asia-Pacific, Bangkok. pp: 88.
- Lilley JH, Philips MJ, Tonguthai K (1992) A review of Epizootic Ulcerative Syndrome (EUS) in Asia. Aquatic Animal Health Research Institute and Network of Aquaculture Centers in Asia-Pacific, Bangkok.
- Kiryu Y, Shields JD, Vogelbein WK, Katorand H, Blazer VS (2003) Infectivity and pathogen city of the Oomycete Aphanomyces invadansin Atlantic menhaden Brevoortia tyrannus. Dis Aquaculture Org 54: 135-146.
- Noga EJ (1993) Fungal and algal disease of temperate freshwater and estuarine fishes, in Stoskopf M.K. (ed.). Fish Medicine. W.B. Saunders, Philadelphia, PA. pp: 278-283.
- Chandra KJ, Basak PK, Das DR (2012) Histopathological observations of farmed carp fingerlings of Mymensingh area. Int Res J Appl Life Sci 1(4): 101-113.
- Camargo MM, Martinez CB (2007) Histopathology of gills, kidney and liver of a Neotropical fish caged in an urban stream. Neotrop Ichthyol 5: 327-336.
- Chinabut S (1990) The histopathology of EUS in Asia. In: Phillips, M.J. and Keddie, H.G. (Eds) Regional Research Programme on Relationship between Epizootic Ulcerative Syndrome in Fish and the Environment. A Report on the Second Technical Workshop, 13-26 August 1990. Network of Aquaculture Centers in Asia-Pacific, Bangkok. pp: 75.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Agriculture and Forest Meteorology Research (ISSN:2642-0449)
- Journal of Astronomy and Space Research
- Journal of Biochemistry and Molecular Medicine (ISSN:2641-6948)
- Proteomics and Bioinformatics (ISSN:2641-7561)
- Journal of Womens Health and Safety Research (ISSN:2577-1388)
- Journal of Genetics and Cell Biology (ISSN:2639-3360)
- Food and Nutrition-Current Research (ISSN:2638-1095)








