2442
Views & Citations1442
Likes & Shares
Keywords: AlS, Thin film, Spray pyrolysis
EXPERIMENTAL DETAILS
Pre-determined solutions containing 0.020 M of aluminium acetate dihydrate (AlC4H6O4.2H2O) and 0.25 M of water-soluble organic compound thiocarbamide (CS(NH2)2) were prepared with solvents; ethylene glycol, deionized water and alcohol. All reagents were ordered from British Drug House England (BDH). The high concentration of thiocarbamide will act as a boost for Sulphur reduction during pyrolysis. Each solution was stirred continuously for an hour until a clear solution was obtained without the inclusion of any advanced agent except for the solution prepared with deionized water in which a drop of H2SO4 was added to prevent precipitation in the mixture. The solution was sprayed on soda lime glasses heated to 350oC at the rate of 2mL/min. The air was used as the carrier gas at a pressure of 30 psi. Spray to target distance was maintained at 30 cm. The resulting AlS thin films were classified as specimens AlG, AlA and AlD, where AlG represents sprayed AlS thin film sample obtained from ethylene glycol, AlA represents sprayed thin film specimen obtained from alcohol and AlD represents sprayed thin film specimen obtained from deionized water. AlG, AlD and AlA were annealed in a muffled furnace at 350oC for one hour. Weight of the films and substrates was measured using Adventurer balance (model: Adventurer Pro AV313 d = 0.0001g.
Characterization of AIS Thin Films
The structural characterization of the samples was done using an X-ray diffractometer with a lynx eye detector using a copper target (Cuα, 1.5418 Å). All X-ray diffraction (XRD) data for the specimens was recorded at current and acceleration voltage of 25mA and 40 kV, respectively. The grain growth analysis of samples /crystallite size was carried out by using Hitachi scanning electron microscope (SEM).
RESULTS AND DISCUSSION
Structural Properties
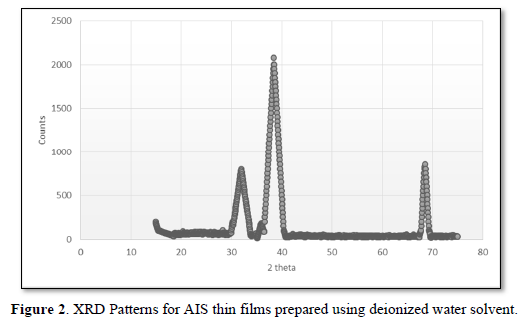
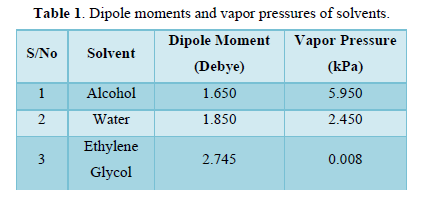
The XRD patterns of AlG, AlD and AlA are shown in Figures 1-3, respectively. The pattern of AlG shows diffraction peaks corresponding to (111), (220) and (311) reflection planes of cubic AlS which are observed at 2θ equaling 38.6º, 48.1º and 57.2º. The broadening observed at the peaks is an evidence of the nanocrystaline nature of the specimen. There was no evidence of secondary phase in the specimen. Crystallite size was calculated using Eq. 1and 3.65 nm was obtained as the crystallite size of AlD. The pattern of AlD shows diffraction peaks at 2θ equaling 30.9º, 38.5º, 48.1º, 56.5º and 69.5º which can be indexed to (101), (111), (112), (220) and (400) respective reflection planes which are the planes of cubic and hexagonal structure of AlS. However, some peaks corresponding to alumiunium oxide (AlO) are observed at 2θ equaling 38.6º and 38.4º. The presence of oxides of Alunimium can be attributed to decomposition of microsized droplets of predetermined mixture during open air spraying. The calculated crystallite size for AlD is 8.160 nm. The pattern of AlA shows peaks at 2θ equaling 30.4º, 33.6º and 38.10 which can be indexed to (111), (101) and (200) reflection planes of cubic and hexagonal phases of AlS respectively. Other peaks that cannot be indexed to planes of AlS were observed in the diffractogram of AlA. A crystallite size of 264.8 nm was obtained for AlA. The variation in crystallite size from crystalline in AlA and AlD to nanocrystalline in AlG due to solvent influence can be explained by taking the vapor pressure and polarity of the solvents into account. Slower evaporation rate induced by lower vapor pressure may have been responsible for small grain size of AlG (Figure 3). The increase in crystallites size with corresponding decrease in polarity can be observed in Figure 3. It can be a suggestion that a reduction in solvent polarity causes loose coiling of bonds, which results in higher aggregate formation. There is an improvement in the structural properties of AlS thin film synthesized using ethylene glycol by post-deposition annealing at 350ºC and without the use of hydrazine which is toxic. Table 1 shows the polarities and vapor pressures of the solvents used in preparing the AlS predetermined solution.
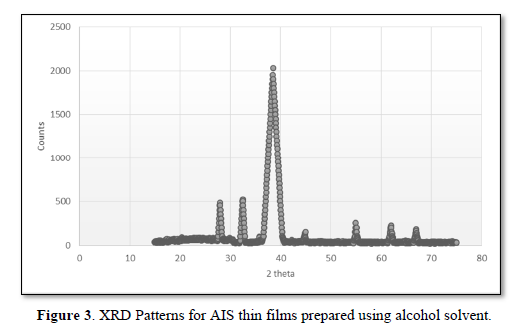
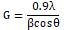 (1)
(1)
Where G is the crystallite size, λ is the CuKα, β is the full width at half maximum and θ is the angle of diffraction.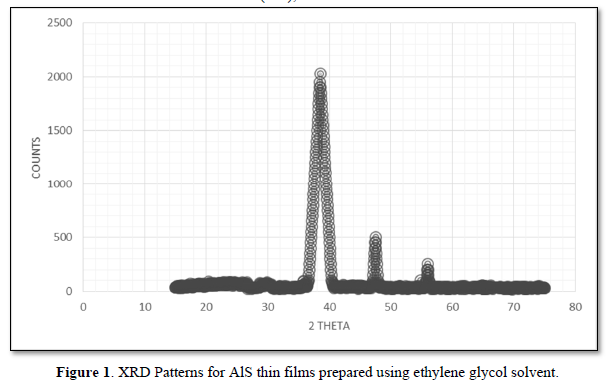
The composition of the solvent with varied AlS thin films was analyzed by energy dispersive X-ray spectroscopy (EDX). The composition of AlG, AlD and AlA are shown in Table 2. The AlS compound is confirmed by the peaks of aluminium (Al) and sulphur (S) in the XRD. It can also be observed that all synthesized films are highly Sulphur-deficient, which may be attributed to affinity of sulphur towards oxygen leading to formation of sulphur (IV) oxide which is lost during pyrolysis [15]. However, the highest atomic percentage of sulphur can be found in specimen AlG, as shown in Table 2, which may be due to drying retardation during the spraying process. Oxygen peaks observed may be due to air used as carrier gas and the carbon peak may be attributed to C=O functional group in AlG.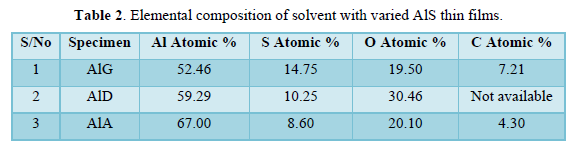
CONCLUSION
In this study, AlS thin films have been synthesized via a chemical technique: spray pyrolysis. The influence of solvents on the structural properties of spray-deposited AlS thin films has been studied. Ethylene glycol, deionized water and alcohol are solvents used in preparation of the predetermined mixture containing aluminium salt and thiocarbamide. The films developed from predetermined mixture with ethylene glycol used as solvent exhibited cubic phase of AlS only, while those developed from deionized water and alcohol showed both cubic and tetragonal phases. It can be suggested from the small crystallite size (2.67 nm) of AlG that the film is nanocrystalline, while AlS thin film synthesized from deionized water has the largest crystallite size of 250.60 nm. The dents are absent in the surface of ethylene glycol-synthesized films. In the case of AlS films prepared from deionized water and alcohol, some voids can be noticed though the particle sizes are more distinguishable compared to those of ethylene glycol.
- Boutebakh FZ, Beloucif A, Aida MS, Chettah A, Attaf N (2017) Zinc molarity effect on Cu2ZnSnS4 thin film properties prepared by spray pyrolysis. J Mater Sci Mater Electron 29: 4089-4095.
- Kassim A, Nagalingam S, Min HS, Karrim N (2010) XRD and AFM studies of ZnS thin films produced by electrodeposition method. Arab J Chem 3(4): 243-249.
- Fan R, Mi Z, Shen M (2019) Silicon based photoelectrodes for photoelectrochemical water splitting. Opt Express 27(4): A184-A196.
- Offor PO, Okorie BA, Ezekoye BA, Ezekoye VA, Ezema JI (2015) Chemical spray pyrolysis synthesis of zinc sulphide (ZnS) thin films via double source precursors. J Ovonic Res 11(2): 73-77.
- Manjulavalli TE, Kannan AG (2015) Structural and optical properties of ZnS thin films prepared by chemical bath deposition method. Int J ChemTech Res 8(11): 396-402.
- Offor PO, Whyte GM, Otung FU, Nnamchi PS, Ude SN, et al. (2019) The characteristics and wettability response of spray-synthesized ZnS films complexed with glycine. Surf Interfaces. 16: 157-163.
- Djelloul A, Adanne M, Larbah Y, Sahraoui T, Zegadi C, et al. (2015) Properties Study of ZnS Thin Films Prepared by Spray Pyrolysis Method. J Nano-Electron Phys 7(4): 1-5.
- Liu W-L, Chen W-J, Hsieh S-H, Yu J-H (2012) Growth Behavior of Nanocrystalline ZnS Thin Films for Solar Cell Using CBD Technique. Procedia Eng 36: 46-53.
- Ashith VK, Gowrish RK (2018) Structural and Optical Properties of ZnS Thin Films by SILAR Technique obtained by acetate Precursor. IOP Conf. Series: Mater Sci Eng 360: 012058.
- Ates A, Yildirim MA, Kundakci M, Astam A (2007) Annealing and light effect on optical and electrical properties of ZnS thin films grown with the SILAR method. Mater Sci Semicon Proc 10(6): 281-286.
- Vishwakarma R (2017) Thickness-Dependent Structural, Electrical, and Optical Properties of ZnS Thin Films Deposited by Thermal Evaporation. Ukr J Phys 62(5): 422-431.
- Ahktar MS, Riaz S, Nazeem S (2015) Optical Properties of Sol-gel Deposited ZnS Thin Films: Spectroscopic Ellipsometry. Mater Today: Proc 2(10): 5497-5503.
- Anila EI, Safeera TA, Raman R (2015) Photoluminescence of nanocrystalline ZnS thin film grown by sol-gel method. J Fluoresc 25(2): 227-230.
- Zaman MB, Chandel T, Dehury K, Rajaram P (2018) Synthesis and characterization of spin-coated ZnS thin films. AIP Conf Proc 1953(1): 100066.
- Falcony C, Aguilar-Frutis MA, Garcia-Hippolito M (2018) Spray Pyrolysis Technique; High-K Dielectric Films and Luminescent Materials: A Review. Micromachines 9: 414.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Advances in Nanomedicine and Nanotechnology Research (ISSN: 2688-5476)
- Journal of Agriculture and Forest Meteorology Research (ISSN:2642-0449)
- Journal of Genomic Medicine and Pharmacogenomics (ISSN:2474-4670)
- Journal of Womens Health and Safety Research (ISSN:2577-1388)
- Journal of Genetics and Cell Biology (ISSN:2639-3360)
- Journal of Veterinary and Marine Sciences (ISSN: 2689-7830)
- Food and Nutrition-Current Research (ISSN:2638-1095)




