Research Article
Measurement of the Volume of the Fetal Adrenal Gland in Two Latin American Maternal Fetal Medicine Units
5951
Views & Citations4951
Likes & Shares
Introduction: The characteristics of the fetal adrenal gland can be evaluated using obstetric ultrasonography with different techniques. In this country and in many others, data on the measurement of their volume by gestational age are not yet available.
Objective: To describe the outcomes of the measurement of the fetal adrenal gland volume by ultrasound evaluation using planimetry (2D) and VOCAL technique (3D) in pregnant women between 18 and 40 weeks of gestation in two Latin American Maternal Fetal Medicine Units.
Methods: Analytical cross-sectional study in which the volume of the fetal adrenal gland was evaluated by ultrasound in pregnant patients, between 18 and 40 weeks of gestation, who attended two Maternal Fetal Medicine services.
Outcomes: 364 patients with mean age of 27.38 years (SD 6.26), with a median gestational age of 30.6 weeks (IQR 24.5- 35.4) were included. The median volume of the fetal adrenal gland was found to have a positive relation between the gestation weeks with the biparietal diameter, the head circumference and the abdominal circumference. Normal values were obtained for the studied population. Percentile and normogram curves were obtained for each gestational age. The interobserver difference in the measurement was calculated. We introduced a smoothed polynomial fractional formula where the volume of the FAG is a function of the gestational age and the gestational age squared.
Conclusions: Fetal adrenal gland is measurable between 18 and 40 weeks of gestation; the planimetry and the VOCAL technique are comparable to its measure, finding a directly proportional relation between its growth and the gestational age. The preliminary volumes of the fetal adrenal gland obtained are comparable to those reported in the literature.
Keywords: Adrenal gland, Fetal, Nomogram
INTRODUCTION
The fetal adrenal gland (FAG) appears at approximately 6 weeks of gestation as a condensation of coelomic epithelium at the cranial end of the mesonephric kidney, occurring as a proliferation of epithelial cells at 8 weeks of gestation [1,2]. With the arrival of ultrasound, many authors have studied the ultrasound appearance and measurement of the normal fetal adrenal gland in the uterus [3-7]. These studies showed that the size of the fetal adrenal gland is positively correlated with gestational age and estimated fetal weight [8]. Besides newborn, similarly, more and more is known about the usefulness of the measurement of the adrenal gland in the fetus [9,10].
The fetal adrenal gland has been the subject of various previous ultrasound investigations, using transabdominal or transvaginal ultrasound. With transvaginal ultrasound, the fetal adrenal gland can be clearly detected from the twelfth week of gestation [5,6,11-13]. There are data in which the gland length was measured in order to establish the parameters of normality [8,13]. Other researchers emphasized the usefulness of volume measurement, not only in 2D ultrasound but also in three-dimensional ultrasound [2,12-14]. Three-dimensional ultrasound (US-3D) could be a method to evaluate the volumes of fetal organs, particularly in cases of asymmetric or irregular structures [15-17]. Thus, the measurement of the three-dimensional volume of the FAG is a good option to have an estimate of this organ, therefore many reference curves for organ volumes have been developed using US-3D [18-20]. However, in the case of the volume of the FAG, there are few available reference curves, which use the planimetry method [1,21]. In fact, to our knowledge, we are not aware of Latin American or local studies that show us the volumetric behavior of the gland in different populations.
The objective of the present study was to determine the volume of the fetal adrenal gland by ultrasonographic evaluation using planimetry (2D), manual VOCAL technique (3D) and automatic VOCAL technique (3D), in pregnant women between 18 and 40 weeks of gestation, in two Latin American Maternal Fetal Medicine units.
METHODS
Analytical cross-sectional study, in which ultrasound measurement of the fetal adrenal gland was performed on pregnant women, between 18 and 40 weeks of gestation, who attended the Maternal Fetal Medicine Units of the Bogota’s Hospital San José and Clinica Colsubsidio 94, from September 2019 to April 2020.
Eligible patients for the study were women older than 18 years, normal pregnant women with a gestational age between 18 and 40 weeks, who attended ultrasound evaluation with singleton pregnancies. Those women with a history of pregnancy pathologies such as threatened abortion and/or preterm delivery, asymptomatic short cervix, intraamniotic infection, hypertensive disorders, gestational diabetes or any abnormality that could alter the placental-fetal unit were excluded. Fetuses with major congenital malformations, fetuses with congenital infection, or fetuses with impaired fetal growth were excluded.
After signing the informed consent and having considered the eligible patient, sociodemographic data were requested. The ultrasound evaluation was performed with the latest generation ultrasound equipment using B mode, 3D with the Accuvix-V10 System (Samsung Medison, Seoul, Korea) with multifrequency transabdominal transducer (4-8 MHz), additionally, it was used the Accuvix-A30 equipment (Samsung Medison, Seoul, Korea) with a multi-frequency transabdominal transducer (4–8 MHz). To obtain the images, the following presets were configured: Smooth, 4/5; FRQ, low; quality, 6; enhance, 16; balance, G> 150; filter, 2; power, 2 dB [22,23].
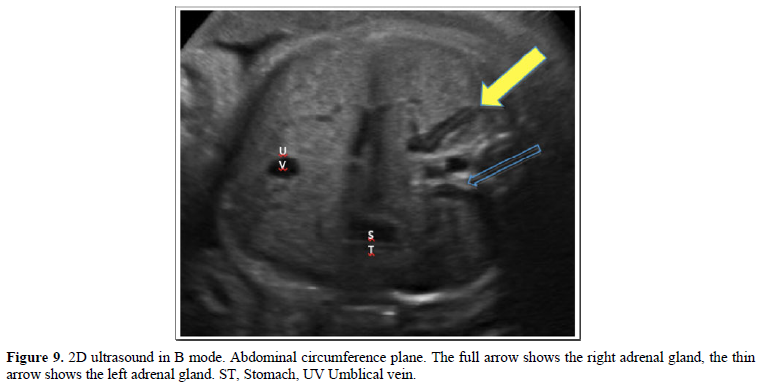
With the patient in the supine position, with the trunk above 30º approximately, the ultrasound evaluation was started according to the ISUOG-AIUM guidelines [24-26], and in compliance with the institutional protocol. After biometry and fetal morphological evaluation, FAG was evaluated in the axial plane, which was identified as a spindle-shape, long linear, echogenic image, with an anechoic halo located on the renal silhouettes, on the side and side of the spine, in the middle of the imaginary triangles formed by the inferior vena cava, the vertebral body and the hepatic angle for the right and for the left the triangle formed by the abdominal aorta, the vertebral body and the splenic angle with the visualization of the gastric chamber, in a plane superior to the measurement of the abdominal circumference [25,26] as described by Nyberg [27]. For the longitudinal plane, the adrenal glands were identified on the respective left and right renal pole, in the renal longitudinal section following the plane of the spine. After identifying and with the dual image, one for the axial plane and the other for the longitudinal plane, we proceed to calculate the volume of the FAG using the ellipse formula: longitudinal diameter x anteroposterior diameter x transverse diameter x 0.523. Same procedure for the contralateral adrenal gland.
Subsequently, with the 3D volumetric transducer, an ultrasound scan was performed at the level of the abdominal circumference with the repair points described above on the FAG, with the following presets: MPR 5 multiplanar reconstruction evaluation mode, scanning sweep angle between 30º and 65º, with an ITE of 1 and a dynamic range of 100. The volumes obtained were stored in the memory of the ultrasound system. For the post-process evaluation of the FAG, data were obtained from the memory of the ultrasound equipment; the three planes obtained in 3D were evaluated. For the post process, the Extended Three-Dimensional imaging (3DXI) software (MEDISON) - 3DXI ® and Virtual Organ Computarized Analyzes-VOCAL ® were used. The upper and lower poles were adjusted at the limits of the gland to be measured and the measurement was started, first manually, recording the taking of 6 contours every 30º each; the volume obtained in cubic millimeters was saved and the histogram option was executed to obtain the value of the medium gray number of the VOCAL image that the equipment returns.
Then, the same process of adjusting the ultrasound measurement parameters was repeated, but the obtaining of the 6 contours was carried out automatically using the general image type function after manual localization of the upper and lower poles. Finally, the histogram was obtained with the medium gray of the image obtained.
All the data were recorded in the collection instrument designed for this purpose. The FAG measurement was recorded: longitudinal, transverse and anteroposterior diameters, manual VOCAL, automatic VOCAL, medium gray for left and right, as well as fetal biometric parameters such as, biparietal diameter, head circumference, occipitofrontal diameter, circumference abdominal and femur length. From these measurements the data of the estimated fetal weight and the percentile were obtained.
We calculated a sample size of 120 pregnant women for each gestational age group (less than 32 weeks, 32 to 36 weeks and more than 37 weeks, total 360 pregnant women) expecting a quadratic relation between gestational age and the volume of the FAG by the different methods for constructing nomograms.
A descriptive statistical analysis was performed, summarizing the qualitative variables with absolute and relative frequencies, and the quantitative ones using measures of central tendency and dispersion according to their statistical distribution. The Shapiro-Wilk test was used to assess normality and the correlation between the volumes of the FAG and the gestational age, and between the volumes of the FAG and the parameters of the biometry and the estimated fetal weight was evaluated, using dispersion diagrams and Spearman's correlation coefficient. The values of the different percentiles of the volumes of the FAG were established according to the gestational age. A generalized linear regression model of the volume of the FAG was constructed based on the weeks of gestation. Finally, we evaluated inter-rater agreement with a Lin coefficient, also plotting the Bland and Altman limits of agreement, with a 95% confidence interval for volume by planimetry and manual VOCAL. The statistical analysis of the information was carried out in Stata V.13.
The study was approved by the research and ethics committee of the participating institutions.
OUTCOMES
A total of 364 patients who underwent obstetric ultrasound evaluation, with gestational ages between 18 and 40 weeks, from two Latin American Maternal Fetal Medicine Units in the period from September 2019 to March 2020 were included. The age of the patients was on average 27.4 (16-45, +/- 6.26 years). Gestational age was calculated by the first ultrasound, presented a minimum value of 18.2 weeks and a maximum of 40.1 weeks (Median = 30.6 weeks RIQ 24.5-35.4). 54.4% (198). 43.7% (159) were nulliparous, 56.3% (205) had at least one living child. 16.8% (61) of the patients had previous gestational losses. 84.1% (306) had a history of vaginal delivery while 15.9% (58) gave birth by caesarean section (Table 1).
The BPD between 40.7 to 114 mm, with a median of 78 mm (IQR 61.1-87.2). The head circumference between 148 to 383.2 mm with a median of 282 mm (IQR 224.3-316.3). The occipitofrontal diameter between 39.9 to 201 mm with a median of 97 mm (IQR 78-108.7). The abdominal circumference between 114.5 to 380 mm with a median of 260 mm (IQR 199 to 310.8). The length of the femur between 20.4 to 77 mm with a median of 57.9 mm (IQR 44.2-66.2). The estimated fetal weight between 236 to 3,770 gr, with a median of 1,605 gr (IQR 683-2581) (Table 2).
The median length of the left FAG was 17.7 mm (IQR 14-21.1) and for the right FAG was 17.9 mm (IQR 14-22). The anteroposterior diameter presented a median for the left FAG of 6.9 mm (IQR 3.9-10) and for the right one of 7.51 mm (IQR 3.9-10.6). The transverse diameter of the left FAG presented a median of 0.72 mm (IQR 0.23-2.07), while for the right FAG was 13.2 mm (IQR 7.25-16.58). The volume by planimetry of the left FAG presented a median of 0.72 cc (IQR 0.23-2.07) and for the right one was 1.04 cc (IQR 0.39-2.07). The manual VOCAL volume for the left FAG presented a median of 0.74 cc (IQR 0.37-2.03), while for the right FAG was 0.97cc (IQR 0.45-2.1). The automatic VOCAL volume presented a median of 0.89 cc (IQR 0.4-2.02) for the left FAG, while for the right one was 1.16 cc (IQR 0.5-2.09). For the left FAG, the medium gray manual VOCAL presented a median of 60.4 (IQR 46.7-84.2), for the right FAG a median of 61.97 was found (IQR 47.36-87.99). The medium gray automatic VOCAL presented a median for the left FAG of 61.8 (IQR 43.99-83.44) and for the right FAG was 62.45 (IQR 47.1-89.03). The difference between these measures was evaluated by means of a student's t test and Wilcoxon's rank test, comparing the left FAG with the right one. No statistically significant differences were found between them (Table 3).
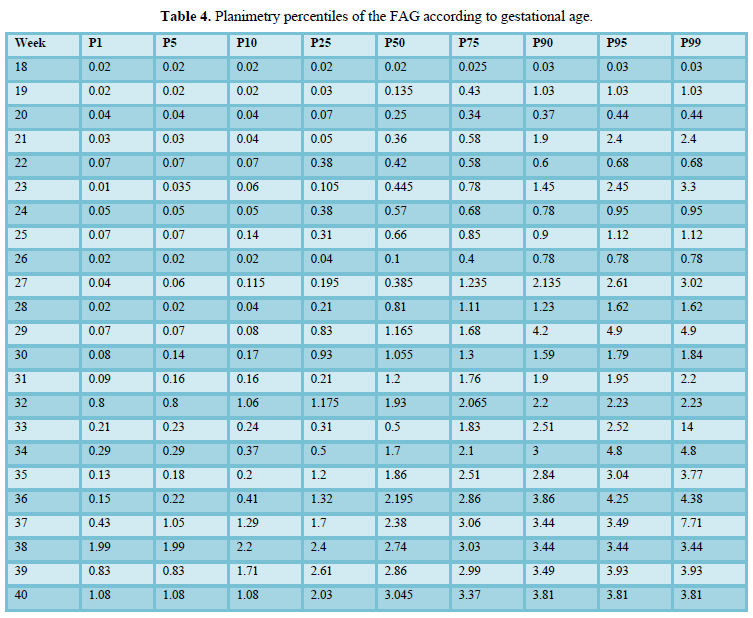
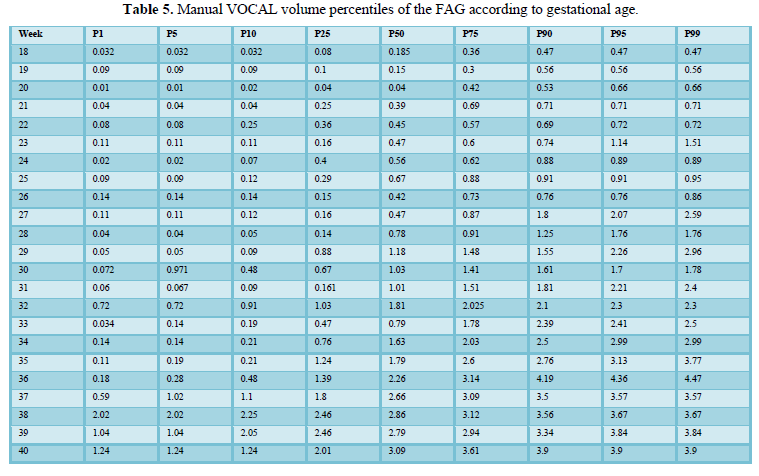
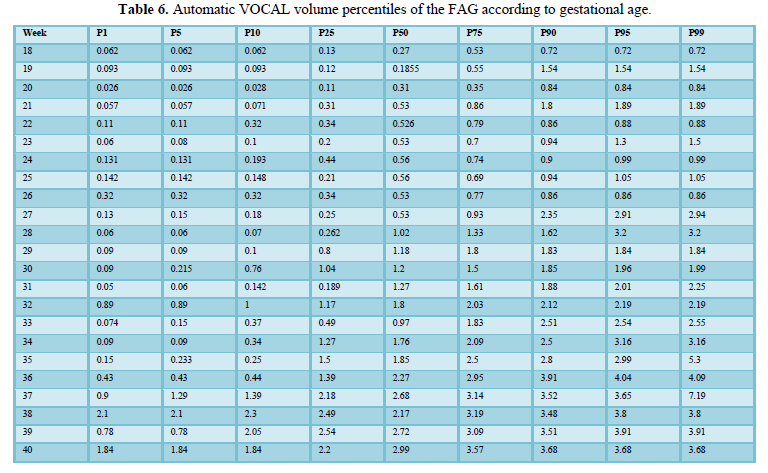
When comparing the median obtained from the volume of the left and right FAG and their average, no statistically significant difference was found using the planimetry volume technique (p-value: 0.62), the manual VOCAL volume (p-value: 0.58) and the automatic VOCAL (p-value 0.75). Spearman's correlation coefficient, for planimetry as automatic and manual VOCAL volumes with respect to gestational age, presented a positive relationship (Spearman’s Rho between 054 to 0.74) (Figures 1-4 and Tables 4-6).
When comparing the median obtained from the volume of the left and right FAG and their average, no statistically significant difference was found using the planimetry volume technique (p-value: 0.62), the manual VOCAL volume (p -value: 0.58) and the automatic VOCAL (p-value 0.75). Spearman's correlation coefficient, for planimetry as automatic and manual VOCAL volumes with respect to gestational age, presented a positive relationship (Spearman’s Rho between 054 to 0.74) (Figures 1-4 and Tables 4-6).
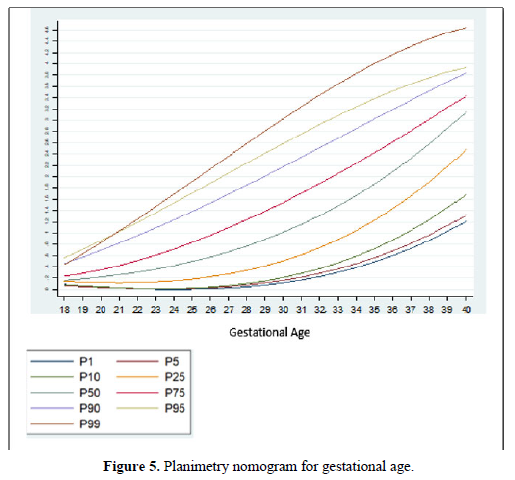
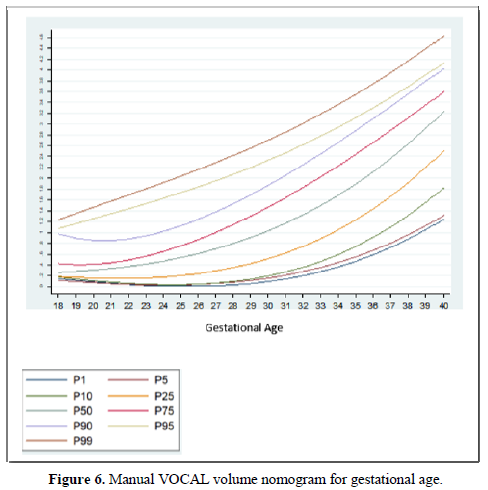
Taking into account that the calculated volumes of the FAG are the same for the left FAG and for the right one, the planimetry nomograms and manual VOCAL are presented (Tables 4 and 5, Figures 5 and 6).
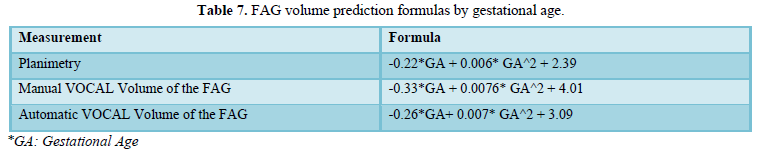
Table 7 shows the formulas for predicting volumes based on gestational age. The automatic VOCAL volume of FAH was determined according to the following formula: -0.26xEG + 0.007 * EG ^ 2 + 3.09 and the planimetry was given by the formula: -0.22 * EG + 0.006 * EG ^ 2 + 2.39. The formula corresponding to the manual VOCAL volume was: -0.33 * EG + 0.0076 * EG ^ 2 + 4.01 (Table 7).
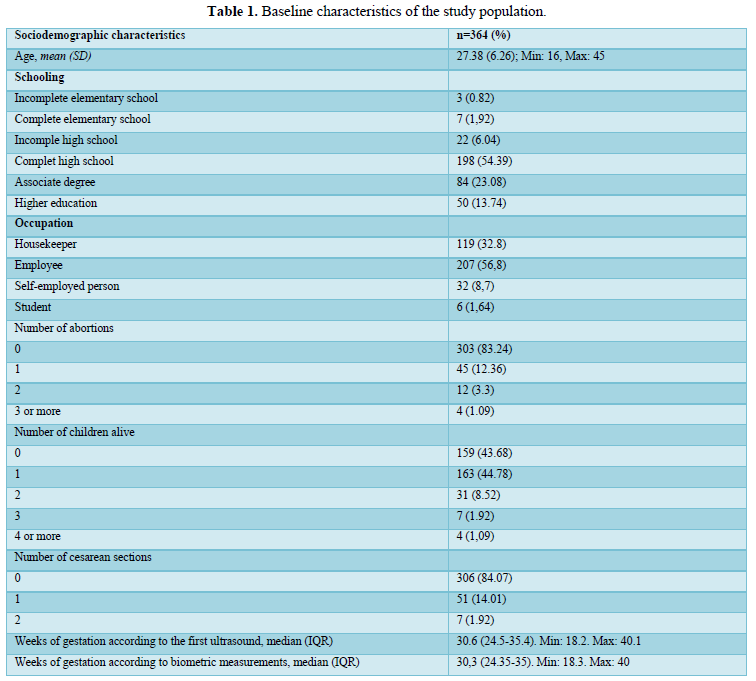
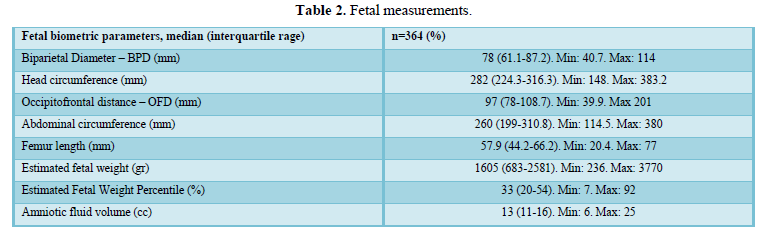
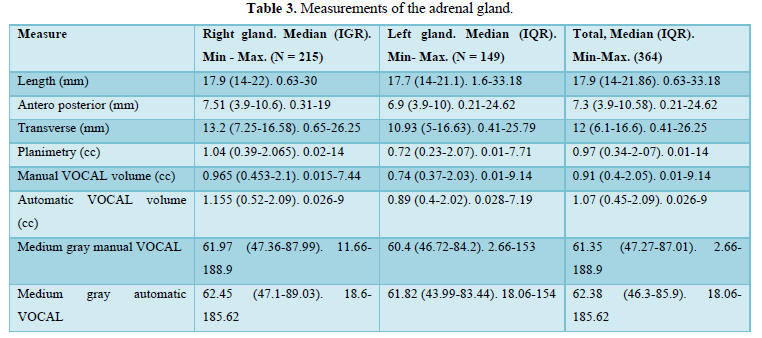
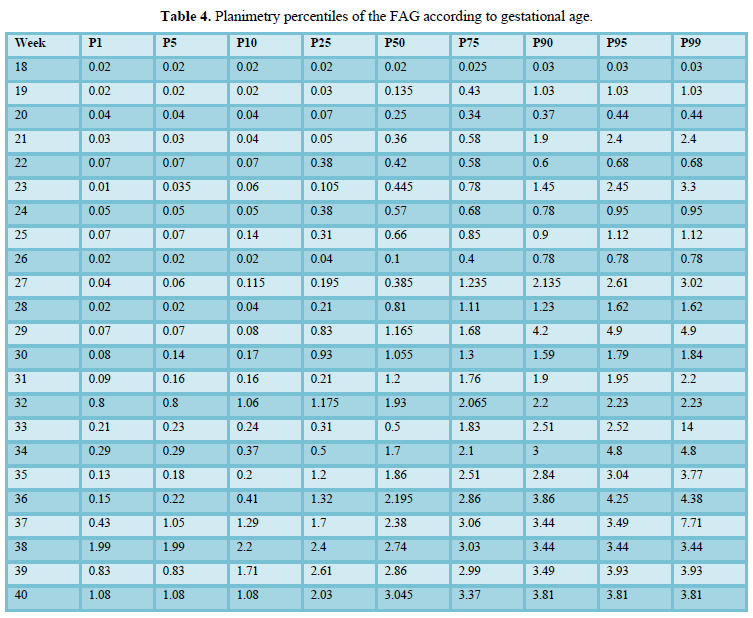
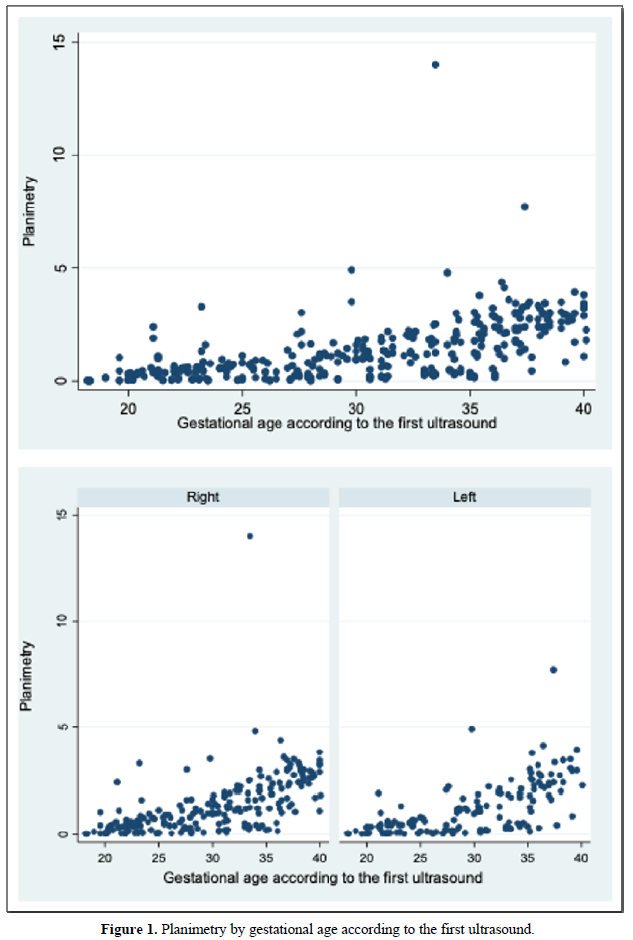
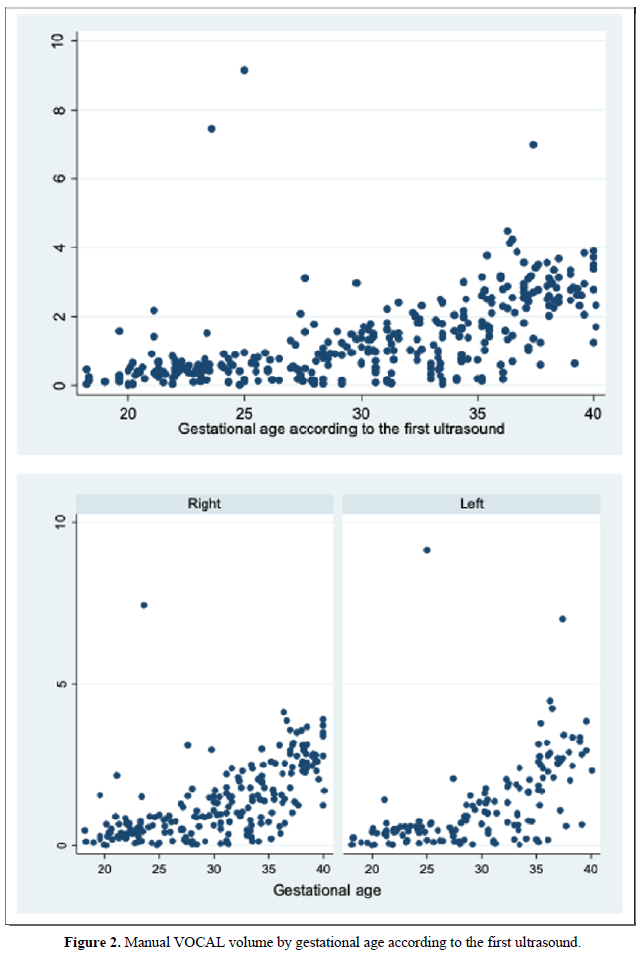
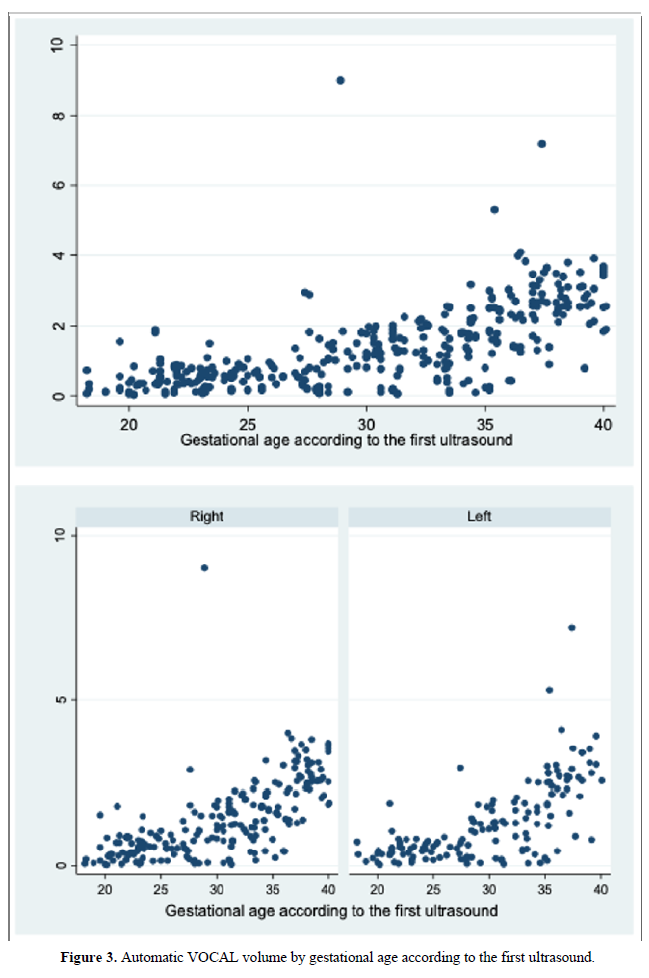
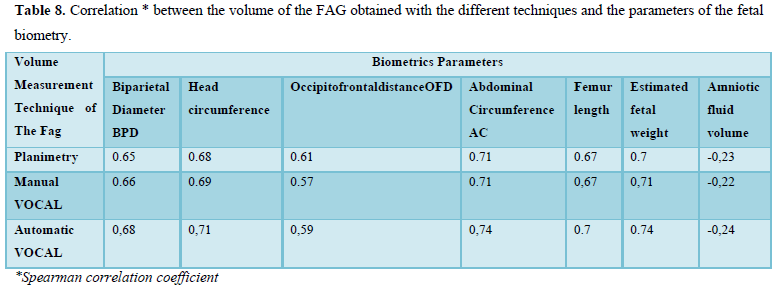


















When evaluating the correlation between volume by planimetry and fetal biometry parameters, an intermediate positive correlation was found (Spearman's rho between 0.6 to 0.74) with biparietal diameter, head circumference, abdominal circumference, femur length and estimated fetal weight. A weak negative correlation with the volume of amniotic fluid. Those results also apply to manual and automatic VOCAL volume (Table 8).
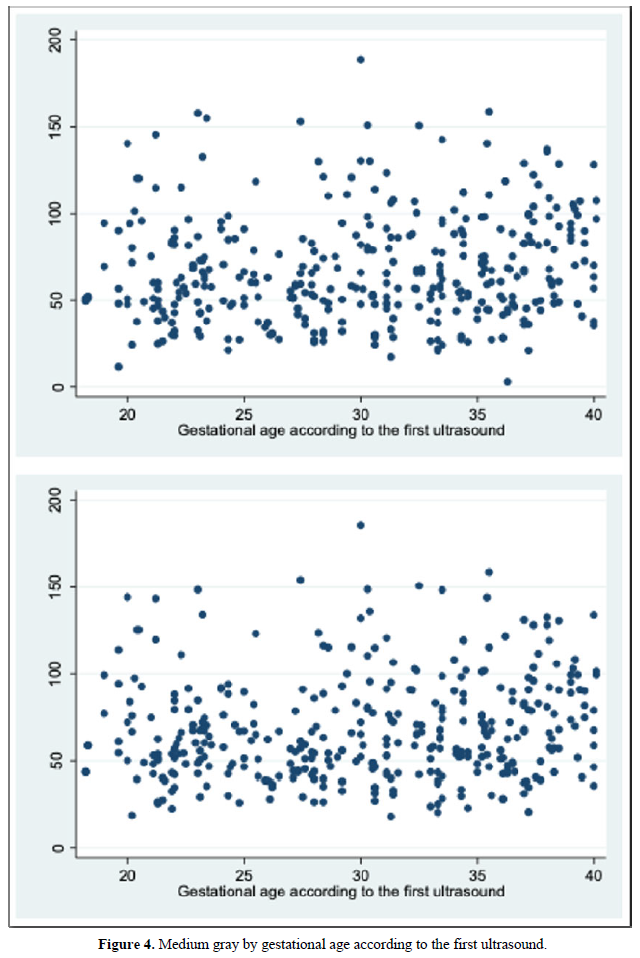
The mean gray measurements for manual and automatic VOCAL volume did not have a statistically significant correlation with the other volume measurements or with weeks of gestation (Spearman's Rho between 0.2 and 0.31).
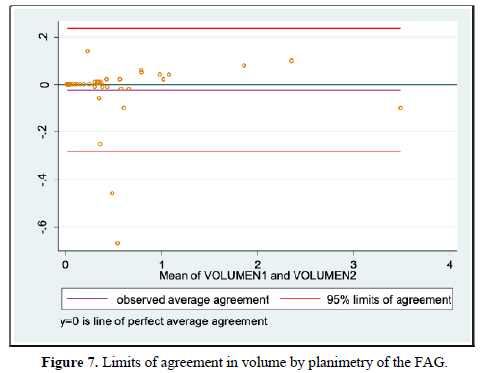
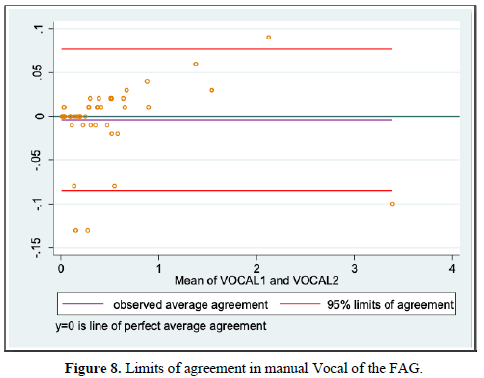
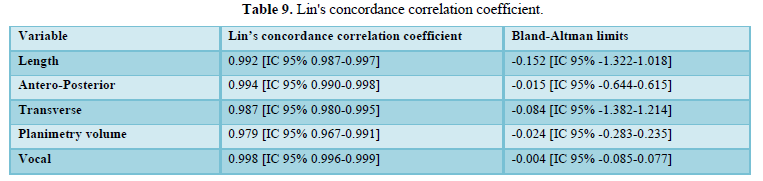
When evaluating the inter-observer agreement for volume by planimetry and manual VOCAL using the Lin coefficient we found a value of 0.979 [95% CI 0.967-0.991] and 0.998 [95% CI 0.996-0.999], respectively. On the other hand, when evaluating the limits of agreement according to Bland and Altman, we found a difference of -0.024 [95% CI -0.283-0.235] for volume by planimetry and -0.004 [95% CI -0.085-0.077] for the VOCAL volume Handbook (Figures 7 and 8 and Table 9).
When evaluating the correlation between volume by planimetry and fetal biometry parameters, an intermediate positive correlation was found (Spearman's rho between 0.6 to 0.74) with biparietal diameter, head circumference, abdominal circumference, femur length and estimated fetal weight. A weak negative correlation with the volume of amniotic fluid. Those results also apply to manual and automatic VOCAL volume (Table 8).
DISCUSSION
Classically, most studies have introduced the planimetry technique as a technique for measuring volume [1,5-7,11,13,28,29,30].However, we know from the anatomical point of view and from the point of view of the sonographic correlation that the FAG have an irregular shape, therefore we could not assume that both FAG have an ovoid shape, however, Chang's studies [21], and Jamigorn et al. [8] found that the measurements of the FAG are directly proportional to the weeks of gestation, with an adequate correlation between the calculation of the volume of FAG by the planimetry technique. The manual VOCAL technique uses the rotational method to generate the volume of the evaluated structures [31]. The volume is generated, not by the sum of the areas as in the planimetry, but by two-dimensional triangulation of a determined number of previously drawn two-dimensional planes. A certain number of planes must be contoured in the rotary path of a hemisphere until completing 180º. The program allows to choose between 4 rotation steps 30º, 15º, 9º and 6º, and then it will rotate the image of the chosen plane [31]. In our measurement of the FAG at 30º, we made the image contour in 6-planes, which allowed us to obtain an image very similar to the actual morphology of the FAG [24-26].VOCAL technique in automatic mode, allows generating the volume of the FAG as in the manual VOCAL technique, generating the contour determining the same number of planes in the 180º rotary path, after adjusting the calipers at the upper pole and lower part of the FAG, in order to delimit the image and obtain its volume. By this method, the operator does not contour the FAG and the equipment automatically dumps the volume [31]. According to the literature review, this is the first time that the automatic VOCAL has been used to assess the volume of the FAG.
We also calculate the mean gray [31]. In our study, we were able to calculate the mean gray for all cases, configuring ourselves in a simple technique that is automatically achieved after having obtained the virtual volume (VOCAL). Although the FAG presents a gray scale that creates different textures in terms of the dynamic range, that is, it has different nuances due to the sono-echographic characteristics, we were unable to demonstrate a correlation between the voxel gray average with respect to two-dimensional / Pixel images. (Spearman's Rho P> 0.05) (Figure 4) [31]. The above would be explained because the automatic VOCAL has some shapes that did not correlate with the actual shape of the FAG. However, it was also not correlated with the manual VOCAL, which is more faithful to the anatomy of the gland. No correlation was found between the mean gray with the GA. We consider it necessary to carry out future studies in order to establish the real utility of the mean gray [32].
Chang [21] obtained one of the first reference curves for the volume of the FAG using 3D ultrasound, in this study the 5th and 95th percentiles reported at 37 weeks of gestation by planimetry were 3.33cc and 5.55cc, respectively. While in our study at the same gestational age they were 1.24 cc for the 5th percentile and 3.9cc for the 95th percentile. The above shows a marked difference between the Taiwanese nomogram, which represents the eastern population, with our Latin-American population, while the study by Helfer [1], using VOCAL, found smaller-volumes, such as 0.248 and 1.15 cc for the 5th and 95th percentile, respectively, -at 37 weeks. Our group evaluated the total volume that includes the fetal area and the definitive area. Jamigorn et al. [8] measured the FAG by 2D ultrasound differentiating the fetal and definitive area by gestational age, however, it did not report FAG volumes.
Among the strengths of our study, we use various methods of measuring the volume of the FAG, which allowed us to make a comparative analysis of the results and determine the effectiveness of the different techniques. In addition, we have a sufficient sample size for each gestational age group, so nomograms were made for the volume of the FAG by gestational age, calculating the different percentiles, thus establishing the expected normal values for our population (Tables 4-6) (Figures 5 and 6). We introduce a smoothed polynomial fractional formula where the volume of the FAG is a function of the gestational age and the gestational age squared (Table 10). When evaluating the volume of the left and right FAG, we show that there are no statistically significant differences between the results obtained (P value> 0.05), so we decided not to distinguish the laterality of the gland when generating the nomograms and correlate with other variables.

We also evaluated inter-observer agreement for volume by planimetry and manual VOCAL using the Lin coefficient, finding a value of 0.979 [95% CI 0.967-0.991] and 0.998 [95% CI 0.996-0.999], respectively, which expresses that more than 95% of the measurements were similar without statistically significant differences between the data. Similarly, using the Bland and Altman method, we found a difference of -0.024 [95% CI -0.283-0.235] for planimetric volume and -0.004 [95% CI -0.085-0.077] for manual VOCAL volume which means the smallest difference between the measurement data. To our knowledge, this difference had not been reported specifically for the measurements of the volumes of the FAG, which makes this measurement reproducible, provided that the observers have adequate training.
The VOCAL technique requires a learning curve to achieve better performance from it, since it is not a routine ultrasound modality and therefore not all specialists in Maternal Fetal Medicine are familiar with it; In addition, its correct processing requires knowledge of both the anatomy of the organ to be evaluated and the ultrasound parameters that must be adjusted before, during and after the process to obtain the best possible image [33-36].
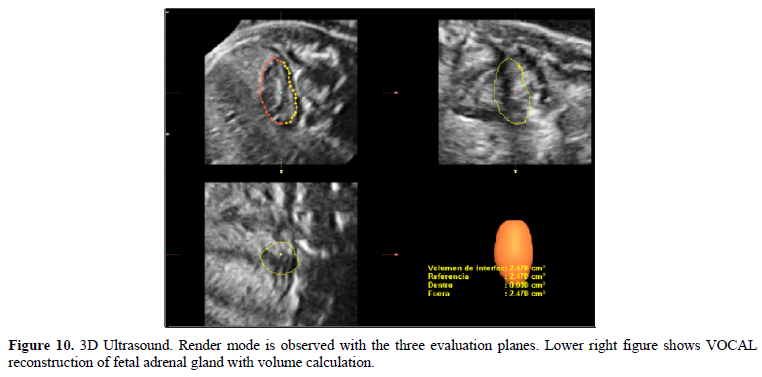
This study has generated a nomogram of the volume of the FAG for a Latin-American population, which has specific differences from some reports in the literature (Figures 9 and 10).


- Helfer TM, Rolo LC, Okasaki NA, Maldonado AADC, Caetano ACR, et al. (2017) Reference range of fetal adrenal gland and fetal zone volumes between 24 and 37 + 6 weeks of gestation by three-dimensional ultrasound. J Matern Fetal Neonatal Med 30(5): 568-573.
- Ferre MS, Jaffe RB (1981) The fetal adrenal gland. Annu Rev Physiol 43: 141-162.
- Iijima S (2018) Sonographic evaluation of adrenal size in neonates (23 to 41 weeks of gestation). BMC Pediatr 18(1): 60.
- Rosenberg ER, Bowie JD, Andreotti RF, Fields SI (1982) Sonographic evaluation of fetal adrenal glands. AJR Am J Roentgenol 139: 1145-1147.
- Jeanty P, Chervenak F, Grannum P, Hobbins JC (1984) Normal ultrasonic size and characteristics of the fetal adrenal glands. Prenat Diagn 4: 21-28.
- Lewis E, Kurtz AB, Dubbins PA, Wapner RJ, Goldberg BB (1982) Real-time ultrasonographic evaluation of normal fetal adrenal glands. J Ultrasound Med 1: 265-270.
- Turan OM, Turan S, Funai EF, Buhimschi, IA, Copel JA, et al. (2007) Fetal adrenal gland volume : A novel method to identify women at risk for impending preterm birth. Obstet Gynecol 109: 855-862.
- Jamigorn M, Phupong V (2017) Nomograms of the whole fetal adrenal gland and fetal zone at gestational age of 16-24 weeks. J Obstet Gynaecol 37(7): 867-871.
- Velaphi SC, Perlman JM (2001) Neonatal adrenal hemorrhage: Clinical and abdominal sonographic findings. Clin Pediatr (Phila) 40: 545-548.
- Al-Alwan I, Navarro O, Daneman D, Daneman A (1999) Clinical utility of adrenal ultrasonography in the diagnosis of congenital adrenal hyperplasia. J Pediatr 135: 71-75.
- Hata K, Hata T, Kitao M (1985) Ultrasonographic identification and measurement of the human fetal adrenal gland in utero. Int J Gynaecol Obstet 23: 355-359.
- Gielchinsky Y, Zvanca M, Akolekar R, Calvo JR, Nicolaides KH (2011) Adrenal gland length in euploid and trisomy 18 fetuses at 11-13 weeks. Prenat Diagn 31:773-777.
- Vuuren SHV, Elias HAD, Stigter RH, Doef RVD, Goldschmeding R, de Jong TP, et al. (2012) Size and volume charts of fetal kidney, renal pelvis and adrenal gland. Ultrasound Obstet Gynecol 40: 659-664.
- Mari G, Uerpairojkit B, Abuhamad AZ, Copel JA (1996) Adrenal artery velocity waveforms in the appropriate and small-for-gestational-age fetus. Ultrasound Obstet Gynecol 8: 82-86.
- Riccabona M, Nelson TR, Pretorius DH, Davidson TE (1995) Distance and volume measurement using three-dimensional ultrasonography. J Ultrasound Med 14: 881-886.
- Riccabona M, Nelson TR, Pretorius DH, Davidson TE (1996) In vivo three-dimensional sonographic measurement of organ volume: Validation in the urinary bladder. J Ultrasound Med 15: 627-632.
- Barreto EQDS, Milani HJF, Junior EA, Haratz KK, Rolo LC, et al. (2010) Reliability and validity of in vitro volume calculations by 3-dimensional ultrasonography using the multiplanar, virtual organ computer-aided analysis (VOCAL), and extended imaging VOCAL methods. J Ultrasound Med 29: 767-774.
- Rolo LC, Nardozza LMM, Araujo Junior E, Nowak PM, Filho JB, et al. (2009) Measurement of embryo volume at 7-10 weeks’ gestation by 3D-sonography. J Obstet Gynaecol 29: 188-191.
- Junior AE, Cavalcante RO, Nardozza LMM, Rolo LC, Ruano R, et al. (2011) Fetal thigh volume by 3D sonography using XI VOCAL: Reproducibility and reference range for Brazilian healthy fetuses between 20 and 40 weeks. Prenat Diagn 31: 1234-1240.
- Ioannou C, Sarris I, Salomon LJ, Papageorghiou AT (2011) A review of fetal volumetry: The need for standardization and definitions in measurement methodology. Ultrasound Obstet Gynecol 38: 613-619.
- Chang CH, Yu CH, Chang FM, Ko HC, Chen HY, et al. (2002) Assessment of fetal adrenal gland volume using three-dimensional ultrasound. Ultrasound Med Biol 28:1383-1387.
- Samsung Medison Accuvix XG Ultrasound Transducer Guide (2020) Available online at: https://www.probomedical.com/samsung-medison-accuvix-xg-ultrasound-transducer-guide/
- Samsung ACCUVIX A30 (2020) Available online at: https://www.samsung.com/latin/support/model/USS-AV30F4U/DE/
- Buitrago M, Beltrán M, Molina S (2014) Guías para la realización de ultrasonidoobstétrico II and III trimestre. Bogotá. D.C. Federación Colombiana de Asociaciones de Perinatología - FECOPEN. Sección de Medicina Materno Fetal, ultrasonidoobstétrico y alto riesgo, 2014. Available online at: http://www.fecopen.org/index.php/proyecto/fecopen/doc_download/1-guias-para-la-realizacion-de-ultrasonido-obstetrico-ii-y-iii-trimestre.
- Salomon LJ, Alfirevic Z, Berghella V, Bilardo C, Andrade EH, et al. (2011) Practice guidelines for performance of the routine mid-trimester fetal ultrasound scan. Ultrasound Obstet Gynecol 37: 116-126.
- AIUM (2019) AIUM Practice Parameter for the Performance of Detailed Second‐ and Third‐Trimester Diagnostic Obstetric Ultrasound Examinations. J Ultrasound Med (2019) 38: 3093-3100.
- Williams L, Philadelphia W (2002) Diagnostic Imaging of Fetal Anomalies. Wilkins LWa, 1stedition, pp: 1102.
- Kim D, Epperson CN, Ewing G, Appleby D, Sammel MD, et al. (2016) Methodology for Using 3-Dimensional Sonography to Measure Fetal Adrenal Gland Volumes in Pregnant Women with and Without Early Life Stress. J Ultrasound Med 35: 2029-2037.
- Turan OM, Turan S, Funai EF, Buhimschi IA, Campbell CH, et al. (2011) Ultrasound measurement of fetal adrenal gland enlargement: an accurate predictor of preterm birth. Am J Obstet Gynecol 204: 311.e1-10.
- Ibrahim MI, Sherif A, El-Kady M, Ellaithy M, Husseiny A, et al. (2015) Can three-dimensional ultrasound measurement of fetal adrenal gland enlargement predict preterm birth? Arch Gynecol Obstet 292: 569-578.
- Sage YH, Lee L, Thomas AM, Benson CB, Shipp TD (2016) Fetal adrenal gland volume and preterm birth: A prospective third-trimester screening evaluation. J Matern Fetal Neonat Med 29: 1552-1555.
- Buhimschi CS, Turan OM, Funai EF, Azpurua H, Bahtiyar MO, et al. (2008) Fetal adrenal gland volume and cortisol/dehydroepiandrosterone sulfate ratio in inflammation-associated preterm birth. Obstet Gynecol 111(3): 15-22.
- Morrison JL (2008) Sheep models of intrauterine growth restriction : Fetal adaptations and consequences. Clin Exp Pharmacol Physiol 35: 730-743.
- McMillen IC, Adams MB, Ross JT, Coulter CL, Simonetta G, et al. (2001) Fetal growth restriction : Adaptations and consequences. Reprod 122: 195-204.
- Merce LT (2010) Ecografía Total. Obstetricia, Ginecología. L. MLS, editor 2010.
- Giraldo SM, Diana AAA, Graterol MA, Olivo JLP, Montero AFS (2019) Three-dimensional Doppler ultrasonography for the assessment of fetal liver vascularization in fetuses with intrauterine growth restriction. Int J Gynaecol Obstet 144(3): 260-264.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Oncology Clinics and Research (ISSN: 2643-055X)
- Journal of Cell Signaling & Damage-Associated Molecular Patterns
- Stem Cell Research and Therapeutics (ISSN:2474-4646)
- Journal of Immunology Research and Therapy (ISSN:2472-727X)
- Journal of Forensic Research and Criminal Investigation (ISSN: 2640-0846)
- Journal of Alcoholism Clinical Research
- Journal of Spine Diseases











