Mini-Review
Hydrodynamics of the G Tube with Physiological Relevance and Clinical Significance
4925
Views & Citations3925
Likes & Shares
This article summarizes the hydrodynamics of the porous orifice (G) tube and demonstrate it has totally different from Poiseuille’s tube. The G tube has negative side pressure gradient that is maximum negative near the inlet and turns gradually positive to become maximum near the exit. Thus, in the G tube suction or absorption of fluid occurs through side holes near the inlet while filtration occurs through holes near the exit. This creates autonomous rapid dynamic magnetic field-like fluid circulation in a surrounding chamber (C) between fluid around the G tube and fluid inside its lumen. The negative side pressure (SP) of G tube creates net negative pressure in C with a direction of flow opposite to that in the G tube.
Both physics and physiological evidence demonstrate that the capillary works as G tube in which the arterial pressure induce negative side pressure gradient that causes absorption by suction not filtration. Starling’s law is thus proved wrong on both forces and equation. Starling’s law being wrong has resulted in many errors and misconceptions on fluid therapy that mislead physicians into giving too much fluid during the resuscitation of shock. The resulting volumetric overload (VO) induces VO shocks (VOS): Sodium-free fluid induces VOS 1 and sodium-based fluid induces VOS 2. An example of VOS 1 is the TURP syndrome known in urology also as hyponatremic shock. This VOS 1 is always mistaken for a known shock and is wrongly treated with further volume expansion that transfers VOS 1 into VOS 2. The later VOS 2 may also complicate fluid therapy of recognized shocks. This is in turn cause ARDS. Both VO and sepsis adversely affect the hydrodynamic of the capillary working as G tube transferring it into Poiseuille’s tube inducing both shock (VOS) and edema of ISF space and of vital organs that cause ARDS or MODS.
Keywords: Capillary physiology, Capillary Interstitial Fluid Transfer, Starling’s law, Hydrodynamic, Hemodynamic, G tube, Poiseuille’s tube
ABBREVIATIONS
G tube: The porous orifice tube; FP: The flow pressure; SP: The side pressure; C: Chamber; VO: Volumetric overload; VOS: Volumetric overload shocks; VOS 1: Volumetric overload shock type 1; VOS 2: Volumetric overload shock type 2; ARDS: The acute respiratory distress syndrome; MODS: the multiple organ dysfunction syndromes
MINI REVIEW
The hydrodynamics of the porous orifice (G) tube was investigated and contrasted to Poiseuille’s tube, and its physiological relevance and clinical importance and significance were reported. The preliminary report was published at Medical Hypothesis in 2001 [1], highlighted in 2017 [2], the physiological evidence in in 2017 [3], and the whole completed [4] and finalized [5] evidence in 2020. The G tube was built on a scale to the capillary ultrastructure with orifice and wide pores mimicking the precapillary sphincter [6] and the wide intercellular clefts [7] that allow the passage of plasma proteins. The later fact nullifies the oncotic pressure in clinical practice.
The G tube investigation of the role of arterial pressure challenges its role in Starling’s law, being a filtration force. The hydrodynamics of Poiseuille’s tube are totally different from G tube. Also, the dynamic pressure of a moving fluid is totally different from the hydrostatic pressure of a stagnant fluid. The dynamic pressure has two components in both the Poiseuille’s tube and the G tube: The flow pressure (FP) that is high positive and works in the direction of the flow. The side pressure (SP) is that exerted on the tube’s wall. It is positive but lower than FP in Poiseuille’s tube, but in the G tube it has a unique effect as it creates negative pressure gradient that turns from high negative near the inlet to high positive near the exit. This induces suction maximum near the inlet where absorption of fluid occurs and turns positive maximum near the exit where filtration occurs. This creates the unique dynamic rapid magnetic field like fluid flow around the G tube between fluid in its lumen and that surrounding it in chamber C- akin to the capillary and interstitial fluid (ISF) space. The G tube hydrodynamic also creates a net negative pressure in a surrounding chamber C. The direction of flow inside C is in the opposite direction to the flow inside the G tube. This explains the negative pressure under the skin of humans and animals as demonstrated by Guyton and Coleman to be -7 cm water [8]. The physiological relevance of the G tube hydrodynamic is this: The G tube hydrodynamics not only proves Starling’s hypothesis [9,10], and law with equations and the Revised Starling’s Principle [11,12] are all wrong, but also provide the correct replacement of the magnetic field like phenomenon of the G tube to explain the capillary-ISF: transfer.
THE PHYSIOLOGICAL RELEVANCE OF THE HYDRODYNAMIC OF THE G TUBE TO NORMAL CAPILLARY PHYSIOLOGY
The provided evidence demonstrates that the hydrodynamic of the G tube is totally different from Poiseuille’s tube. This is relevant to the physiological function of capillary regarding the capillary-ISF transfer. When Starling proposed his hypothesis on the formation of oedema in 1886 and 1896 [9,10], he assumed that the capillary works as Poiseuille’s tube of uniform diameter and its hydrostatic pressure induced by the high arterial pressure is responsible for filtration of fluid higher over the proximal part of the capillary near the inlet. He wrongly thought the capillary works as Poiseuille’s tube. It was discovered >80 years later in 1967 that the capillary has a narrow orifice; the precapillary sphincter [6]. He also wrongly assumed that absorption of fluid is induced by the oncotic pressure of plasma proteins as he thought that the capillary wall is impermeable to albumin. It was also discovered in 1967 that the capillary has wide pores of intercellular slits that allow molecules larger than plasma proteins to pass through [7] - hence nullify oncotic pressure in vivo. Starling’s hypothesis was made into a law later. In fairness to Professor Starling, who was a great physiologist, he never wrote any equations nor proposed a law. Here we demonstrate that Starling’s law is wrong on both of its forces [1-5], and the equations must be also wrong. This affirms the principle of what is built on wrongdoing must also be wrong. Both physics [1,2,4,5] and physiological [3] evidence demonstrate that the capillary works as G tube in which the arterial pressure induce negative side pressure gradient that causes absorption by suction not filtration that is maximum near the inlet. This is based on the hydrodynamic of the G tube summarized here. It has also been demonstrated that the oncotic pressure does not exist in vivo as the capillary has wide intercellular slit pores that allow molecules larger than plasma proteins to pass through it [7]. Starling’s law is thus wrong on both of its forces and the equations must also be wrong. It is time to say farewell: “goodbye Starling’s law, hello G tube” [13].
THE PATHOLOGICAL SIGNIFICANCE OF HYDRODYNAMIC OF THE G TUBE WHEN THE CAPILLARY ACTS AS POISEUILLE’S TUBE INDUCING VOS AND ISF EDEMA OF ARDS
Starling’s law being wrong has resulted in many errors and misconceptions on fluid therapy [14]. These errors mislead physicians [15] into giving too much fluid during the resuscitation of shock, the acutely ill patient and prolonged major surgery. The resulting volumetric overloads (VO) induce VO shocks (VOS) [16, 17] which cause the acute respiratory distress syndrome (ARDS) [18, 19]. VOS are two types depending on the type of fluid inducing it: Sodium-free fluid induces VOS 1 and sodium-based fluid induces VOS 2. Examples of VOS 1 are the transurethral resection of the prostate (TURP) syndrome [20] known in urology also as hyponatremic shock [21]. These are induced by massive absorption of 3.5-5 l in one hour of 1.5% Glycine used as irrigating fluid for the TURP surgery and/or excessive infusion of 5% Glucose. This VOS 1 is always mistaken for the known hemorrhagic or septicemic shock and is wrongly treated with further volume expansion using crystalloids and/or colloids that transfer it into VOS 2. The later VOS 2 may also complicate fluid therapy of recognized shocks during therapy using crystalloids and/or colloids and blood. This is in turn causing ARDS [18, 19] or the multiple organ dysfunction syndromes (MODS). Both VO and sepsis adversely affect the hydrodynamic of the capillary working as G tube transferring it into Poiseuille’s tube causing both shock (VOS) and oedema of ISF space particularly in the subcutaneous tissue and of vital organs that characterize and cause ARDS or MODS [18,19].
CONCLUSION
The G Tube has totally different hydrodynamic from Poiseuille’s tube. The G tube has negative side pressure gradient that is maximum negative near the inlet and turns gradually positive to become maximum near the exit. Thus, in the G tube suction or absorption of fluid occurs through side holes near the inlet while filtration occurs through holes near the exit. This creates autonomous rapid dynamic magnetic field-like fluid circulation in a surrounding chamber (C) between fluid around the G tube and fluid inside its lumen. The negative SP of G tube creates net negative pressure in chamber (C) with a direction of flow opposite to that in the G tube.
Both physics and physiological evidence demonstrate that the capillary works as G tube in which the arterial pressure induce negative side pressure gradient that causes absorption by suction not filtration. Starling’s law is thus proved wrong on both forces and equation. Starling’s law being wrong has resulted in many errors and misconceptions on fluid therapy that mislead physicians into giving too much fluid during the resuscitation of shock. The resulting volumetric overload (VO) induce VO shocks (VOS): Sodium-free fluid induce VOS 1 and sodium-based fluid induce VOS 2. Examples of VOS 1 is the TURP syndrome known in urology also as hyponatremic shock. This VOS 1 is always mistaken for a known shock and is wrongly treated with further volume expansion that transfer VOS 1 into VOS 2. The later VOS 2 may also complicate fluid therapy of recognized shocks. This is in turn cause ARDS or the multiple organ dysfunction syndrome (MODS). Both VO and sepsis adversely affect the hydrodynamic of the capillary working as G tube transferring it into Poiseuille’s tube inducing both shock (VOS) and edema of ISF space and of vital organs that cause ARDS or MODS.
Figure 1 shows Poiseuille's tube hydrodynamic with positive side pressure along the entire length of the tube causing fluid to filter out maximum near the inlet and minimum near the exit. This is what Starling had based his hypothesis on regarding the hydrostatic pressure causing filtration maximum near the orifice. This will be compared to the hydrodynamic of the G tube (Figure 2) built on a scale to capillary ultrastructure of pre-capillary sphincter and intercellular clefts making wide capillary pores that allow the passage of molecules larger than plasma proteins.
Figure 2 shows the hydrodynamic of the G tube's with side pressure gradient lower at the inlet where it is negative and turns into positive pressure maximum near the exit, with visible magnetic field-like circulation around it seen at your top right-hand quarter of the photo-based on which and other photos shown below, the diagram showing the G-C circulation was drown (Figure 3). There is negative side pressure gradient over the proximal part of G tube not shown here but is shown in Figure 4.
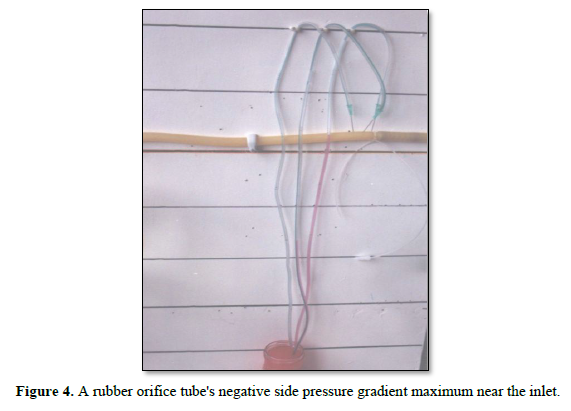
Figure 4 shows a rubber orifice tube's negative side pressure gradient maximum near the inlet, turning into positive pressure maximum near the exit as shown in Figure 2, with visible magnetic field-like circulation around it seen at your top right-hand quarter of the photo- based on which and other photos shown here, the diagram showing the G-C circulation was drawn (Figure 4). This rubber orifice tube was also used for measuring the flow pressure (FP) and side pressure (SP) which is dynamic components of the lumen pressure (LP) induced by the proximal pressure (PP) - akin to arterial pressure.
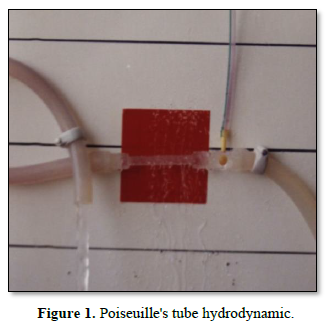

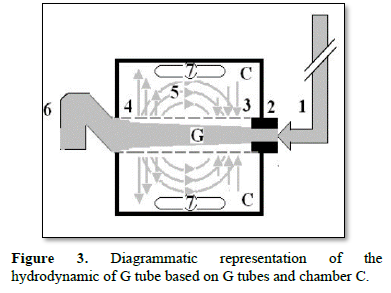



Figure 4 shows a rubber orifice tube's negative side pressure gradient maximum near the inlet, turning into positive pressure maximum near the exit as shown in Figure 2, with visible magnetic field-like circulation around it seen at your top right-hand quarter of the photo- based on which and other photos shown here, the diagram showing the G-C circulation was drawn (Figure 4). This rubber orifice tube was also used for measuring the flow pressure (FP) and side pressure (SP) which is dynamic components of the lumen pressure (LP) induced by the proximal pressure (PP) - akin to arterial pressure.
Figure 3 shows a diagrammatic representation of the hydrodynamic of G tube based on G tubes and chamber C. This 38-years old diagrammatic representation of the hydrodynamic of G tube in chamber C is based on several photographs. The G tube is the plastic tube with narrow inlet and pores in its wall built on a scale to capillary ultra-structure of precapillary sphincter and wide inter cellular cleft pores, and the chamber C around it is another bigger plastic tube to form the G-C apparatus. The chamber C represents the ISF space. The diagram represents a capillary-ISF unit that should replace Starling’s law in every future physiology, medical and surgical textbooks, and added to chapters on hydrodynamics in physics textbooks. The numbers should read as follows:
- The inflow pressure pushes fluid through the orifice
- Creating fluid jet in the lumen of the G tube**.
- The fluid jet creates negative side pressure gradient causing suction maximal over the proximal part of the G tube near the inlet that sucks fluid into lumen.
- The side pressure gradient turns positive pushing fluid out of lumen over the distal part maximally near the outlet.
- Thus, the fluid around G tube inside C moves in magnetic field-like circulation [5]. Taking an opposite direction to lumen flow of G tube.
- The inflow pressure 1 and orifice 2 induce the negative side pressure creating the dynamic G-C circulation phenomenon that is rapid, autonomous, and efficient in moving fluid and particles out from the G tube lumen at 4, irrigating C at 5, then sucking it back again at 3.
- Maintaining net negative energy pressure inside chamber C.
**Note the shape of the fluid jet inside the G tube (Cone shaped), having a diameter of the inlet on right hand side and the diameter of the exit at left hand side (G tube diameter). I lost the photo on which the fluid jet was drawn, using tea leaves of fine and coarse sizes that run in the center of G tube leaving the outer zone near the wall of G tube clear. This may explain the finding in real capillary of the protein-free (and erythrocyte-free) sub-endothelial zone in the Glycocalyx paradigm. It was also noted that fine tea leaves exit the distal pores in small amount maintaining a higher concentration in the circulatory system- akin to plasma proteins.
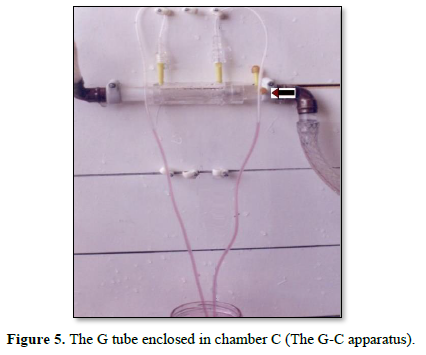
Figure 5 shows the G tube enclosed in chamber C (The G-C apparatus). The negative side pressure of G tube also creates a negative pressure in C shown here to suck the red water from a jar 300 mm below G tube into the manometers.
Figure 6 shows the G tube enclosed in a rubber chamber (C) which is sucked in not Ballooned out demonstrating the negative pressure in (C) akin to the negative pressure measured by Guyton and Colman [17] using a subcutaneous implanted chamber- a remarkable fact that cannot be explained by Starling’s forces.




- Ghanem AN (2001) Magnetic field-like fluid circulation of a porous orifice tube and its relevance to the capillary-interstitial fluid circulation: Preliminary report. Med Hypotheses 56: 325-334.
- Ghanem KA, Ghanem AN (2017) The proof and reasons that Starling’s law for the capillary-interstitial fluid transfer is wrong, advancing the hydrodynamics of a porous orifice (G) tube as the real mechanism. Blood Heart Circ 1: 1-17.
- Ghanem KA, Ghanem AN. (2017) The Physiological Proof that Starling’s Law for the Capillary-Interstitial Fluid Transfer is wrong: Advancing the Porous Orifice (G) Tube Phenomenon as Replacement. Open Acc Res Anatomy. 1: 2.
- Ghanem AN (2020) The Correct Replacement for the Wrong Starling’s law is the Hydrodynamic of the Porous Orifice (G) Tube: The Complete Physics and physiological Evidence with Clinical Relevance and Significance. Cardio Open Acc 5: 1-9.
- Ghanem AN (2020) Final proof Starling’s law wrong and G tube correct replacement: New results and critical analytical criticisms of landmark articles. (Under Consideration).
- Rhodin JA (1967) The ultra-structure of mammalian arterioles and pre-capillary sphincters. J Ultrastruct Res 18: 181-222.
- Karnovesky MJ (1967) The ultra-structural basis of capillary permeability studied with peroxidase as a tracer. J Cell Biol 35: 213-236.
- Guyton AC, Coleman TG (1968) Regulation of interstitial fluid volume and pressure. Ann N Y Acad Sci 150: 537-547.
- Starling EH (1886) Factors involved in the causation of dropsy. Lancet 2: 1266-1270, 1330-1334, 1406-1410.
- Starling EH (1896) On the absorption of fluids from connective tissue spaces. J Physiol 19: 312-326.
- Woodcock TE, Woodcock TM (2012) Revised Starling equation and the glycocalyx model of transvascular fluid exchange: An improved paradigm for prescribing intravenous fluid therapy. Br J Anaesth 108: 384-394.
- Michel CC, Woodcock TE, Fitz‐Roy EC (2020) Understanding and extending the Starling principle. Acta Anaesthesiol Scand 64: 1032-1037.
- Ghanem AN (2020) Goodbye Starling's law, hello G tube. J Urol Nephrol 5(1): 000175.
- Ghanem AN (2018) The Adult Respiratory Distress Syndrome: Volumetric Overload Shocks in Patho-Aetiology, Correcting Errors and Misconceptions on Fluid Therapy, Vascular and Capillary Physiology. Surg Med Open Acc J 2: 2.
- Ghanem AN (2020) What are Misleading Physicians into giving too much Fluid during Resuscitation of Shock and Surgery that Induces ARDS and/or AKI?” Asploro J Biomed Clin Case Rep 3: 90-98.
- Ghanem AN (2020) Volumetric Overload Shocks (VOS) in Surgical Patients. Open Access J Surg 11: 555810.
- Ghanem AN (2020) Volume Kinetic Shocks in Clinical Practice. Clin Surg J 3: 1-5.
- Ghanem AN (2020) Volumetric Overload Shocks Cause the Acute Respiratory Distress Syndrome: The Plenary Evidence on Patho-Aetiology and Therapy. Op Acc J Bio Sci Res 4: 1.
- Ghanem AN (2020) Volumetric Overload Shocks Cause the Acute Respiratory Distress Syndrome: Building the Bridge Between Physics, Physiology, Biochemistry, and Medicine. Biomed J Sci Tech Res 29: 22197- 22209.
- Ghanem AN, Ward JP (1990) Osmotic and metabolic sequelae of volumetric overload in relation to the TUR syndrome. Br J Urol 66: 71-78.
- Harrison III RH, Boren JS, Robison JR (1956) Dilutional hyponatremic shock: Another concept of the transurethral prostatic resection reaction. J Urol 75: 95-110.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Oral Health and Dentistry (ISSN: 2638-499X)
- Journal of Cancer Science and Treatment (ISSN:2641-7472)
- International Journal of Internal Medicine and Geriatrics (ISSN: 2689-7687)
- Archive of Obstetrics Gynecology and Reproductive Medicine (ISSN:2640-2297)
- Journal of Infectious Diseases and Research (ISSN: 2688-6537)
- Journal of Allergy Research (ISSN:2642-326X)
- Journal of Neurosurgery Imaging and Techniques (ISSN:2473-1943)








