Case Report
Cholestyramine in Thyrotoxicosis: A Boon and A Bane
4617
Views & Citations3617
Likes & Shares
Cholestyramine is a well-tolerated therapy for Hypercholesterolemia. There are reports stating the effectiveness of cholestyramine as an adjunct therapy to antithyroid medications, particularly in refractory thyrotoxicosis. This resin is useful in rapid reduction of serum thyroid hormone levels. Though relatively a safe drug, most adverse reactions caused by this is because it can affect the absorption of various nutrients and vitamins. Hypernatremia induced by cholestyramine is very rare. Here we report a case where Cholestyramine was successfully used to treat thyrotoxic storm but induced Hypernatremia, which was resolved by withdrawing the drug.
Keywords: Cholestyramine, Thyrotoxic storm, Hypernatremia with cholestyramine
CASE PRESENTATION
A 57-year-old gentleman, known diabetic for 15 years on oral medications, and hypertensive, presented to the emergency room with complaints of heaviness of chest, associated with breathing difficulty and palpitations. His examination revealed him to be in atrial fibrillation. His ECG confirmed atrial fibrillation with fast ventricular rate (159 bpm) with diffuse ST depression in all leads. An emergency echocardiogram revealed moderate to severe LV systolic dysfunction (LVEF-32%) with regional wall motion abnormality of the left Anterior Descending artery territory. No clot, effusion or vegetation were seen. A possibility of acute coronary syndrome associated with heart failure with reduced ejection fraction was considered and he was admitted in the coronary care unit (CCU) for further evaluation and management.
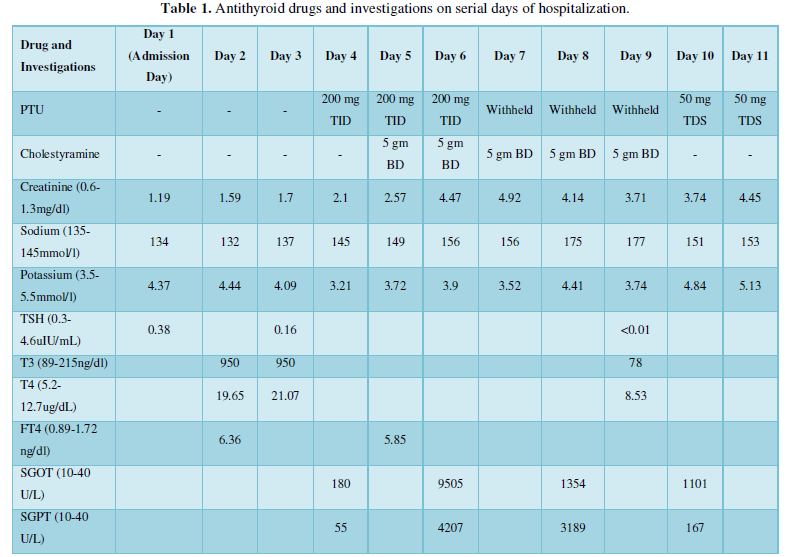
Following admission, he was managed with rate control medications (Amiodarone), inotropic agents, coronary vasodilators and non-invasive ventilatory support. In view of elevated cardiac enzymes and persistent decompensated heart failure in spite of anti-failure medication, the need for assessing the coronary and ad hoc revascularization was considered. Hence, he was electively intubated and was taken up for emergency coronary angiogram and revascularization. Coronary angiogram revealed severe triple vessel disease. Subsequently intra-aortic balloon pump (IABP) was inserted along with ionotropic supports. High risk CABG (coronary artery bypass grafting) and need for ECMO (extracorporeal membrane oxygenation) support was considered. His initial blood reports had shown suppressed TSH and hence full thyroid functions were done. This showed elevated thyroid hormones (Table1).
Hyperthyroidism was worsening the cardiac dysfunction and the possibility of the cardiac dysfunction being thyrotoxic cardiomyopathy was also thought of. Hence on the second day of admission, an endocrinology consultation was sought. On examination, he had no goiter or eye signs suggestive of thyroid associated orbitopathy (Graves’ Disease).
Hence the possibilities considered were Amiodarone induced thyrotoxicosis or apathetic Graves’ disease. There was a past history of this gentleman being on certain medications for thyroid illness and was off the same for over six months, but the family could not confirm if this was thyroxine supplements or anti-thyroid drugs.
Clinically he was in thyrotoxic storm as per the Burchs and Wartofsky criteria (Table 2) and hence a multidisciplinary team of cardiologists, cardiac surgeons, Endocrinologists and Cardiac anesthesiologists evaluated him and decided to defer CABG till thyroid status stabilized.
He was treated with diuretic infusions, Heparin, dual antiplatelets, statins, and antibiotics along with inotropic support. He developed recurrent AF and was reverted with cardioversion and also boluses of Amiodarone.
Medical management of the thyrotoxic storm was started with Inj. Hydrocortisone 100 mg Q6h, and Tab. Propylthiouracil 200 mg TID. He could not be given beta blockers in view of poor LV function. He had mild elevation of hepatic transaminases at baseline and within 48 hours of starting PTU he developed hepatitis with very high transaminase levels. In view of possible drug induced hepatitis, PTU was stopped. Renal functions were also worsening. At this point it was decided to start Cholestyramine as thioamides could not be used to control thyrotoxic storm. Cholestyramine was started at 5 gm twice daily. Liver functions improved after stopping PTU. His thyroid functions also improved rapidly (Table 1). However, during the treatment of thyroid storm he developed hypernatremia which was an unexpected challenge. On the day of starting cholestyramine therapy his sodium was 145mEq/l and this kept increasing steadily despite adequate fluid intake and urine output, reaching 177mEq/l on the fifth day. His renal functions started deteriorating from the day 2 of admission and creatinine was steadily going up despite having urine output of around 3 lt daily. The possibility of cholestyramine causing hyponatremia was thought of and hence this was stopped. He could not be given much free water owing to his heart failure and hence he was also initiated on slow low efficiency dialysis (SLED). Within a day hypernatremia resolved and gradually his heart failure, liver enzymes and renal parameters improved. Inotropic support was slowly tapered and his ventilatory support was also reduced.
He was rechallenged with PTU at 50 mg thrice daily, which he tolerated well. On the 20th Day of admission, he became hypothyroid (TSH-10). His TSH receptor antibody was sent in order to confirm Grave’s disease, but this was negative. A final diagnosis of thyrotoxic storm worsening coronary artery disease, AF and ventricular function was made.


DISCUSSION
Hyperthyroidism is a set of disorders that involve excess synthesis and secretion of thyroid hormones by the thyroid gland, which leads to the hypermetabolic condition of thyrotoxicosis [1,2]. Hyperthyroidism becomes a thyrotoxic storm when its manifestations become life-threatening, which apparently happens in 1-2% of patients with clinically overt hyperthyroidism. It is defined as a life-threatening exacerbation of hyperthyroid state with one or more organ dysfunction [3].
Thyrotoxic storm can be life threatening, particularly when there is a precipitating event.
In our patient the precipitating event may have been the acute coronary event as well as the acute iodine load from Amiodarone. He had biochemical thyrotoxicosis with very high FT4: 6.36ng/dl (0.89 - 1.72). He had no clinical features of toxicity except atrial fibrillation. If at all he had underlying thyroid dysfunction, this was probably exaggerated to a thyroid storm by the Iodine load in Amiodarone (37% of Iodine). A patient taking a standard 200 mg daily dose of Amiodarone ingests 75 mg of organic iodine each day. Subsequent de-iodination through drug metabolism results in the daily release of approximately 6 mg of free circulating iodine which equates to 20-40 times higher than the average daily iodine intake which is approximately 0.15 mg (150 mcg). This increase in iodine delivery and uptake increases thyroid hormone production and release. This condition is known as type I Amiodarone induced thyrotoxicosis (Seen in patients with underlying Graves’ disease or goiter). In addition, Amiodarone can also result in Type II Amiodarone induced thyrotoxicosis which causes actual thyroid destruction and release of stored hormones.
In our patient thyrotoxic storm was worsening the cardiac dysfunction, and hence had to be controlled quickly. Beta Blockers could not be used and antithyroid drugs had to be withheld in view of increasing liver enzymes. At this point cholestyramine came as a rescue drug, which helped control the storm quickly. Thyroid hormone is metabolized mainly in the liver, where it is conjugated to glucuronides and sulfates. These conjugation products are then excreted into the bile through enterohepatic circulation. A fraction of conjugated products is deconjugated in the intestine, and free hormones are reabsorbed. In thyrotoxicosis, enterohepatic circulation of thyroid hormone is increased. Cholestyramine decreases reabsorption of thyroid hormone from enterohepatic circulation [4]. Doses up to 12 grams in 3 divided daily doses have been used for 4 weeks [5].
Cholestyramine being a cationic resin, exchanges chloride anions in the small bowel with anions such as bile acids and bicarbonates. Each gram of resins contains 4 meq of chloride. This chloride is excreted by the kidney. Thus, each gram of cholestyramine will add about 2.5 mosmol of anion and an equal amount of cation to the renal solute load [6].
To make a cation-exchange resin with a stronger acidity, water-insoluble styrene is used to prepare the polymer, which is sulfonated to make it hydrophilic. Styrene divinyl benzene copolymer (DVB) is an anion exchanger that contains carboxyl or sulfate groups with hydrogen, potassium, and sodium as counterions. DVB is also used to cross-link the polymer, and the counterion of the sulfate group (SO3) is generally sodium [7]. Thus, exchange of sodium takes place, leading to hypernatremia.
Unless extra water is provided to compensate for both the increase in solute load and the hydration of cholestyramine, the obligatory loss of fluid will lead to hyperchloremia and hypernatremia and a hyperosmolar state will develop. When cholestyramine therapy is indicated, it is therefore important to modify the diet in order to decrease the solute load or provide additional water [8].
In our patient, cholestyramine proved to be beneficial in treating thyrotoxicosis but resulted in unwarranted complication of hypernatremia, which was recognized and treated successfully.
CONCLUSION
We present this case to highlight the usefulness of cholestyramine in thyrotoxic storm and at the same time to be on the lookout for complications like hyperchloremic nonanion gap metabolic acidosis and hypernatremia.
ACKNOWLEDGEMENT
We wish to acknowledge the support from our colleagues Dr. Philip Finny & Dr. Jibily Joy from the department of Endocrinology department, Dr. Praveen & Dr. Leena from the department of cardiology, Dr. Kannan and Dr. Benson from CTVS, all the staff of coronary care unit and all our other colleagues from BCMCH who helped in looking after this patient.
- Blick C, Nguyen M, Jialal I (2020) Thyrotoxicosis. In: Stat Pearls [Internet]. Treasure Island (FL): Stat Pearls Publishing; 2021 Jan.
- Doubleday AR, Sippel RS (2020) Hyperthyroidism. Gland Surg 9(1):124-135.
- Douglas SR, Burch HB, Cooper DS, Greenlee MC, Laurberg P, et al. (2016) 2016 American Thyroid Association guide lines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid 26(10): 1343-1421.
- Yang Y, Hwang S, Kim M, Lim Y, Kim MH, et al. (2015) Refractory Graves’ Disease Successfully Cured by Adjunctive Cholestyramine and Subsequent Total Thyroidectomy. Endocrinol Metab (Seoul) 30(4): 620-625.
- Lee SL, Ananthakrishnan S (2020) Hyperthyroidism and Thyrotoxicosis. Treatment & Management. Drugs and disease, Medscape. Available online at: https://emedicine.medscape.com/article/121865-overview
- Ha J, Jo K, Kang B, Kim MH, Lim DJ (2016) Cholestyramine Use for Rapid Reversion to Euthyroid States in Patients with Thyrotoxicosis. Endocrinol Metab (Seoul) 31(3): 476-479.
- Mahajan S (2001) Encyclopedia of Materials: Science and Technology, (1st edition) Pergamon, Elsevier.
- Primack WA, Gartner LM, Mcgurk HE, Spitzer A (1977) Hyperchloremia and Hypernatremia Associated with Cholestyramine Therapy. Pediatr Res 11: 520.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Immunology Research and Therapy (ISSN:2472-727X)
- Journal of Alcoholism Clinical Research
- Ophthalmology Clinics and Research (ISSN:2638-115X)
- Journal of Spine Diseases
- International Journal of Anaesthesia and Research (ISSN:2641-399X)
- Journal of Clinical Trials and Research (ISSN:2637-7373)
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)


