Research Article
Twenty-Two Year Survival in 15-Year-Old Female with a Recurrent Posterior Fossa Ependymoma Treated with Antineoplastons
3009
Views & Citations2009
Likes & Shares
Purpose: Posterior fossa Ependymoma typically arise in the floor of the fourth ventricle and can extend through the foramen of Luschka or foramen of Magendie causing hydrocephalus. They can present with headache, double vision, cranial nerve dysfunction, and torticollis. Treatment normally consists of surgery and radiation therapy (RT). This original article discusses the use of intravenous (IV) and oral Antineoplastons A10 and AS2-1 (ANP therapy) in the treatment of a 15-year-old female with a recurrent posterior fossa ependymoma.
Material and Methods: This African-American female was first seen at the Burzynski Clinic (BC) on January 21, 1997, four years following completion of subtotal resection, RT, and chemotherapy elsewhere. MRI of the brain showed a 13.3 cm2 enhancing posterior fossa mass on coronal images. Physical exam demonstrated hearing loss in the right ear and a well-healed suboccipital scar. Lansky score was 90%. The patient was enrolled in BT-24, a Phase II protocol utilizing ANP therapy in the treatment of ependymomas.
Results: Tumor response to ANP therapy was measured utilizing sequential magnetic resonance images (MRIs) of the brain, with and without gadolinium contrast. Following 2.5 years of IV and oral ANP therapy, a partial response (PR) was obtained. There has been no evidence of tumor progression now for 22 years.
Conclusions: ANP is an effective treatment for some posterior fossa ependymomas and for a variety of other low- and high-grade brain tumors. Multiple Phase II protocols utilizing ANP have now been completed and its impact on the treatment of brain tumors has been widely published.
Keywords: Brain tumor, Ependymoma, Posterior fossa ependymoma, Recurrent posterior fossa ependymoma, IV Antineoplaston therapy, Oral Antineoplaston therapy, Phase II clinical study
Abbreviations: A10: Antineoplaston A10; AS2-1: Antineoplaston AS2-1; ANP Therapy: Antineoplastons A10 and AS2-1 (IV and oral); Atengenal: Antineoplaston A10; Astugenal: Antineoplaston AS2-1; CR: Complete Response; CT: Computed Tomography; BC: Burzynski Clinic; BRI: Burzynski Research Institute; FLAIR: Fluid-Attenuated Inversion Recovery; isoPG: Phenylacetylisoglutamine; IV: Intravenous; MRI: Magnetic Resonance Imaging; OR: Objective Response; PD: Progressive Disease; PG: Phenylacetylglutamine; PN: Phenylacetate; PR: Partial Response; RT: Radiation Therapy; SCE: Spinal Cord Ependymoma; SD: Stable Disease; WHO: World Health Organization
INTRODUCTION
Ependymomas are rare glial tumors that account for approximately 7% of all intracranial neoplasms in adults [1]. However, ependymomas are among the most common brain tumors in children. More than half of these cases occur in children under the age of five [2]. They are most commonly found within the posterior fossa (60%), followed by the supratentorial region (30%) and spinal canal (10%) [3]. The World Health Organization (WHO) classification system of ependymomas ranges from WHO Grade I-III depending on the location and histologic appearance [1]. Ependymomas in the adult population typically, present when they are larger than 4 cm2 in size and often contain cystic components, whereas ependymomas in the pediatric population are usually solid and smaller in size at presentation [4].
Intracranial ependymomas are most commonly WHO Grade II and occur in both children and adults. Infratentorial ependymomas most often arise in children as posterior fossa tumors [4] while supratentorial ependymomas are typically seen in younger to middle age adults [5]. The MRI features of ependymomas include low T1, high T2, and iso- to high fluid-attenuated inversion recovery (FLAIR) signal intensity due to their increased myxoid component [6]. Ependymomas are heterogeneously enhancing masses that demonstrate avid enhancement of the soft tissue components with adjacent areas of little to no enhancement. Anaplastic ependymoma, which is typically supratentorial, is a WHO Grade III intracranial tumor that is more aggressive with a higher proliferative rate and a greater tendency for infiltration and dissemination into the cerebrospinal fluid [7]. It is a rare tumor and its criteria for histopathologic grading is not well established, but includes marked hypercellularity, nuclear atypia, and increased mitotic activity. Supratentorial ependymoma can present with headache, weakness, visual field loss, and seizures.
In the posterior fossa, ependymomas typically arise in the floor of the fourth ventricle and the infiltrative nature of these tumors can result in extension from the fourth ventricle through the foramen of Luschka or foramen of Magendie [8]. Posterior fossa ependymoma can present with headache, double vision, other cranial nerve dysfunction, and torticollis (turning the head to one side).
Spinal cord ependymoma (SCE) is a rare tumor that is most commonly low-grade. Complete surgical resection has been established as first-line treatment and can be curative. However, SCEs tend to recur when complete tumor resection is not possible. Evidence supporting the use of adjuvant radiation and chemotherapy is not definitive [9]. Spinal cord ependymoma may present with weakness and/or loss of sensation, pain, and bowel or bladder dysfunction. Primary therapy for ependymomas is surgery with or without radiation therapy (RT). In a follow-up study of 101 cases, the postoperative prognosis was poor in intracranial tumors. RT increased the survival time but was not curative. The prognosis in patients with spinal ependymomas was much better, with 75% of the patients surviving for at least 10 years. Anaplastic histology has been associated with a poor prognosis [10].
We present here the case of an African-American female with a recurrent posterior fossa ependymoma who, after surgery, RT and chemotherapy elsewhere, presented to the Burzynski Clinic (BC) when she was 15 years of age.
METHODS
In 1992, a 10-year-old female presented to her pediatrician because of headaches, dizziness, and blurred vision. In February of 1992, Computed tomography (CT) scan of the brain demonstrated a large mass extending from the fourth ventricle into the foramens of Lushka and Magendie with accompanying obstructive hydrocephalus. On February 17, 1992, 90-95% of the tumor was removed via a suboccipital craniotomy. Histologic examination of the surgical specimen revealed an ependymoma. Post-operative magnetic resonance imaging (MRI) of the brain, with and without gadolinium contrast, revealed a small residual enhancing lesion in the posterior fossa with associated enhancement of the meninges. From April 7, 1992 through May 20, 1992, 5400 cGy RT was given to the fourth ventricle and the upper cervical spine. MRI performed on June 11, 1992 showed a reduction in the enhancement previously seen. From June 29, 1992 through February 13, 1993, the patient received chemotherapy (Vincristine, Carboplatin, VP-16, Ifosfamide, and Mesna). On January 13, 1993, MRI showed post-operative changes, a decrease in the size of the ventricles, and no residual enhancement. However, on January 3, 1997 (4 years later), MRI of the brain and spine showed a recurrent tumor in the prepontine space and suprasellar area with extension across the midline.
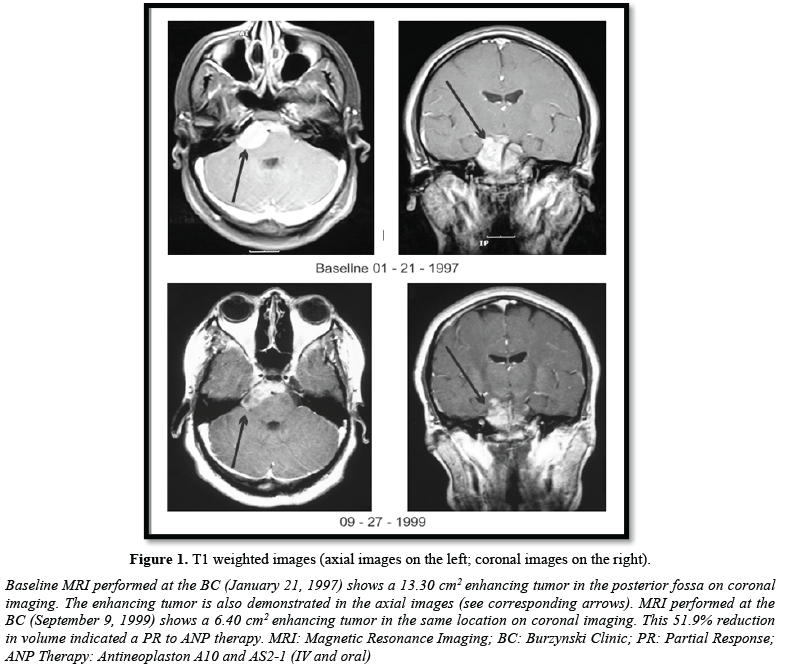
The patient was seen at the Burzynski Clinic (BC) on January 21, 1997. She was a 15-year-old African-American high school student. Physical exam revealed hearing loss in the right ear and a well-healed suboccipital scar. Lansky score was 90%. MRI of the brain performed on the same day showed a 13.3 cm2 enhancing posterior fossa tumor on coronal images (Figure 1). On January 23, 1997, the patient was enrolled in BT-24, a “Phase II Study of Antineoplastons A10 and AS2-1 (ANP therapy) In Patients with Ependymomas”. This was a single arm, open label study in which patients with persistent, progressive, or recurrent ependymoma received gradually increasing doses of intravenous (IV) A10 and IV AS2-1 via subclavian catheter and infusion pump, until a maximum tolerated dose of each component was achieved. When an objective response (OR) was achieved, ANP therapy (IV or oral) was continued 6 to 12 months. Disease progression, unacceptable toxicity, physician decision, or patient request resulted in termination of ANP therapy.
The eligibility criteria for BT-24 included age > 6 months, histologically confirmed ependymoma, baseline MRI of the brain demonstrating a persistent, progressive, or recurrent ependymoma, which was ≥ 5 mm in size, Lansky/Karnofsky score of 60-100%, and life expectancy of > 2 months. All study subjects or their guardians read, understood, and signed a written informed consent prior to enrollment. Outcome criteria were 1) OR and 2) survival. The safety and tolerance of ANP therapy in patients with ependymomas were also investigated.
To determine OR, tumor size was measured utilizing sequential MRIs of the brain, with and without gadolinium enhancement. Tumor size was calculated as the product of the two greatest perpendicular diameters as determined by imaging. Response criteria were as follows: a complete response (CR) indicated complete disappearance of all enhancing tumor while a partial response (PR) indicated a > 50% reduction in enhancing tumor size. CR and PR required a confirmatory MRI performed at least four weeks after the initial finding. Progressive disease (PD) indicated a > 25 % increase in enhancing tumor size, or new enhancing disease, while stable disease (SD) did not meet the criteria for PR or PD [11]. All MRIs were reviewed by a prominent outside radiologist.

RESULTS AND DISCUSSION
BT-24 began accruing patients in July 1966 and was closed in October 2000 due to slow accrual of patients. Nine patients, in total, were accrued, of which 5 (55.6%) were female. All study subjects were seen at the BC. The median age was 7.3 years (range of 1.6 to 35.7 years). Three patients were not evaluable. Of the six evaluable patients, one patient (presented here) had a PR, two patients had SD, and 3 patients had PD. All evaluable patients survived at least 6 months; four patients survived at least 12 months; three patients survived at least 24 months; two patients survived at least 48 months; and one patient survived more than 60 months.
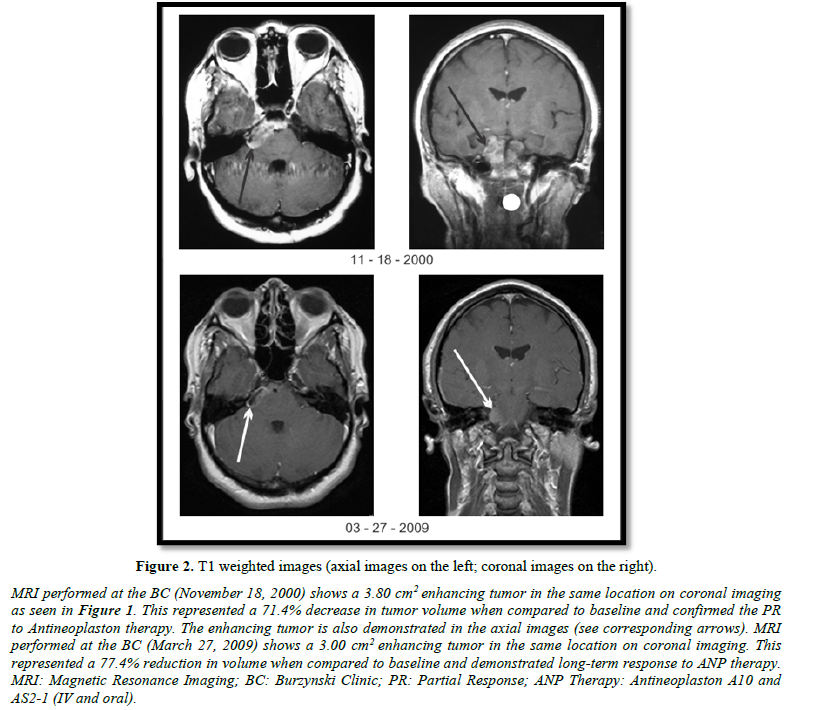
As previously discussed, the patient presented here achieved a PR on ANP therapy and has experienced long-term survival. Baseline MRI (January 21, 1997) showed an enhancing lesion measuring 13.30 cm2 on coronal images (Figure 1). The patient was treated with IV ANP therapy per BT-24. The dosages of A10 and AS2-1 were gradually increased to 16.21 g/kg/d and 0.43 g/kg/d, respectively. Serial MRIs showed some decrease of the contrast-enhancing tumor on coronal images through 11/12/1998. At that time, the enhancing tumor measured 10.98 cm2, an 18.1% decrease from baseline. The patient was started on Antineoplaston A10 and AS2-1 capsules (0.5 mg) at that time because repeated subclavian catheter infections prevented further IV ANP therapy. The dosage of ANP therapy was gradually increased to two capsules (1.0 mg) of each formulation qid. Oral therapy was stopped on January 10, 2003. On September 27, 1999 (Figure 1), November 18, 1999 (Figure 2), and March 27, 2009 (Figure 2), the enhancing tumor measured 6.40 cm2, 3.80 cm2, and 3.00 cm2 on coronal images, respectively, which represented a 51.9%, a 71.4%, and a 77.4% decrease in tumor size. A PR had been achieved by September 27, 1999 and was stable 9.5 years later based on MRI evidence. On August 30, 2021 an email was received from the patient stating that she was doing very well with no evidence of tumor progression. She gave written permission for the use of the figures presented in this article. Without further MRI evidence, we can assume, at a minimum that the PR achieved has lasted 22 years. There is a reasonable possibility that the patient has achieved a CR. The patient experienced one grade 3 serious adverse event (SAE), which was possibly related to ANP therapy, and recovered fully.

Antineoplaston research began in 1967, when significant deficiencies were noticed in the peptide content of the serum of patients with cancer compared with healthy persons. Initially Antineoplastons were isolated from the blood and later from urine [12]. Subsequent studies of the isolated Antineoplastons demonstrated that Antineoplaston A10 (A10, Atengenal) and Antineoplaston AS2-1 (AS2-1, Astugenal) were the most active Antineoplastons. The chemical name of A10 is 3-phenylacetylamino-2,6-piperidinedione. It consists of the cyclic form of L-glutamine connected by a peptide bond to phenylacetyl residue. When given orally, Antineoplaston A10 resists the attack of gastric enzymes. In the small intestine, under alkaline conditions, 30% is digested into phenylacetylglutamine (PG) and phenylacetylisoglutaminate (isoPG) in a ratio of approximately 4:1. The mixture of synthetic PG and isoPG in a 4:1 ratio, dissolved in sterile water constitutes A10 IV injection. Further metabolism of A10 results in phenylacetate (PN). Both metabolites PG and PN have anticancer activity. The mixture of PN and PG in a 4:1 ratio, dissolved in sterile water constitutes AS2-1 IV injection [13]. In 1988, following the completion of Phase I studies of A10 and AS2-1, Dr. S. R. Burzynski developed the Phase II protocol designated BT-3 and titled “Therapy of Primary Brain Tumors with Antineoplaston A10 and Antineoplaston AS2-1” The objectives of this protocol were to determine 1) the effectiveness of these Antineoplastons in the control of primary brain tumors and 2) the toxicity of these Antineoplastons in patients with primary brain tumors. On October 4, 1991, three members of the NIH Cancer Therapy Evaluation Program, with an invited neuropathologist and an invited neuroradiologist, visited Dr. Burzynski at the BC to review seven selected brain tumors from Phase I and Phase II studies (including BT-3). Following through review, five definite or “possible” CRs were identified [14]. BT-3 led to further Phase II clinical studies, which involved a more aggressive use of ANP therapy, including continuous infusions of higher dose A10 and AS2-1 utilizing ambulatory infusion pumps, as was the case in BT-24.
CONCLUSIONS
In this report, we have presented a 15-year-old female with a recurrent posterior fossa ependymoma, who received ANP therapy, had resolution of her tumor-induced signs and symptoms, achieved a PR by MRI, and has survived for 22 years. ANP therapy has been utilized in in a variety of low- and high-grade brain tumors under the Burzynski Research Institute’s (BRI’s) IND # 43,742. Multiple Phase II protocols have been completed and the impact of ANP on the treatment of brain tumors has been widely published [15-51].
- McLendon RE, Wiestler OD, Kros JM, Korshunov A, Hg HK (2016) Ependymoma. WHO classification of tumors of the central nervous system. (4th ed), International Agency for Research on Cancer, Lyon.
- Koeller KK, Sandbeg GD (2002) Cerebral Intravascular neoplasms: Radiographic-pathologic correlation. Radiographics 22: 1473-1505.
- Howlander N, Noone AM, Krapcho M, Bishop K (2016) SEER cancer statistics review, 1975-2014. National Cancer Institute.
- Mermuys K, Jeuris W, Vanhoenacker PK, Van Hoe L, D’Haenens P (2005) Supratentorial ependymoma. AFIP Archives-Best Cases from the AFIP.
- Smith AB, Smirniotopoulos JG, Horkanyne-Szakaly I (2013) From the radiologic pathology archives: Intravascular neoplasms: Radiologic-pathologic correlation. Radiographics 33: 21-43.
- Vitanza NA, Partap S (2015) Pediatric ependymoma. J Child Neurol 31: 1354-1366.
- McLendon RE, Wiestler OD, Kros JM, Korshunov A, Hg HK (2016) Subependymoma. WHO classification of tumors of the central nervous system. (4th ed), International Agency for Research on Cancer, Lyon.
- Plaza MJ, Borja MJ, Altman N, Saigal G (2013) Conventional and advanced MRI features of pediatric intracranial tumors: Posterior fossa and suprasellar tumors. Am J Roentgenol 200: 1115-1124.
- Celano E, Salehani A, Malcom JG, Reinertsen E, Hadjipanayis CG (2016) Spinal cord ependymoma: A review of the literature and case series of ten patients. J Neurooncol 128(3): 377-386.
- Massimino M, Miceli R, Giangaspero F, Boschetti L, Modena P, et al. (2016) Final results of the second prospective AIEOP protocol for pediatric intracranial ependymoma. Neuro Oncol 18 (10): 1451-1460.
- Wen PK, Macdonald DR, Reardon DA, Timothy Fc, Sorensen AG, et al. (2010) Updated response criteria for high-grade gliomas: Response Assessment in Neuro-Oncology (RANO) working group. J Clin Oncol 28(11): 1963-1972.
- Burzynski SR (1976) Antineoplastons: Biochemical defense against cancer. Physiol Chem Phys 8: 275-279.
- Burzynski SR (1986) Synthetic antineoplastons and analogs. Drugs Future 11: 679-688.
- Hawkins MG, Friedman MA (1992) National Cancer Institute’s evaluation of unconventional cancer treatments. J Natl Cancer Inst 84: 1699-1702.
- Burzynski SR (2006) Targeted Therapy for Brain Tumors. In: Yang AV, editor. Brain Cancer Therapy and Surgical Interventions. Nova Science Publishers, Inc, New York.
- Burzynski SR (2006) Treatments for astrocytic tumors in children: Current and emerging strategies. Ped Drugs, 8, 167-168.
- Burzynski SR (2007) Recent clinical trials in diffuse intrinsic brainstem glioma. Cancer Ther 5: 379- 390.
- Burzynski SR, Conde AB, Peters A, Saling B, Ellithorpe R, et al. (1999) A Retrospective Study of Antineoplastons A10 and AS2-1 in Primary Brain Tumors. Clin Drug Invest 18: 1-10.
- Burzynski SR, Weaver RA, Bestak M, Lewy RI, Janicki TJ, et al. (2003) Phase II study of Antineoplastons A10 and AS2-1 (ANP) in children with recurrent and progressive multicentric glioma: A preliminary report. Neuro Oncol 5: 358.
- Burzynski SR, Lewy RI, Weaver R, Janicki T, Jurida G, et al. (2004) Long-term survival and complete response of a patient with recurrent diffuse intrinsic brain stem glioblastoma multiforme. Integ Cancer Ther 3: 257-261.
- Burzynski SR, Weaver R, Lewy R, Janicki T, Jurida G et al. (2004) Phase II study of antineoplaston A10 and AS2-1 in children with recurrent and progressive multicentric glioma: A preliminary report. Drugs R&D 5: 315-326.
- Burzynski SR, Weaver R, Bestak M, Janicki T, Jurida G et al. (2004) Phase II studies of antineoplastons A10 and AS2-1 (ANP) in children with atypical teratoid/rhabdoid tumors (AT/RT) of the central nervous system: A preliminary report. Neuro Oncol 6: 427.
- Burzynski SR, Weaver R, Bestak M, Janicki T, Szymkowski B, et al. (2004) Treatment of primitive neuroectodermal tumors (PNET) with antineoplastons A10 and AS2-1 (ANP): Preliminary results of phase II studies. Neuro Oncol 6: 428.
- Burzynski SR, Weaver RA, Janicki, T, Szymkowski B, Jurida G, et al. (2005) Long-term survival of high-risk pediatric patients with primitive neuroectodermal tumors treated with Antineoplastons A10 and AS2-1. Integ Cancer Ther 4: 168-177.
- Burzynski SR, Janicki, TJ, Weaver RA, Burzynski B (2006) Targeted therapy with Antineoplastons A10 and AS2-1 of high grade, recurrent, and progressive brainstem glioma. Integ Cancer Ther 5: 40-47.
- Burzynski S, Janicki T, Burzynski G, Marszalek A (2014) Long-term survival (>13 years) in a child with recurrent diffuse pontine gliosarcoma: A case report. J Ped Hematol Oncol 36: 433-439.
- Burzynski SR, Janicki TJ, Burzynski GS, Marszalek A (2014) A phase II study of antineoplastons A10 and AS2-1 in children with high-grade glioma: Final report (Protocol BT-06) and review of recent trials. J Cancer Ther 5: 565-577.
- Burzynski SR, Janicki TJ, Burzynski GS (2014) A phase II study of antineoplastons A10 and AS2-1 in adult patients with recurrent glioblastoma multiforme: Final report (Protocol BT-21). J Cancer Ther 5: 946-956.
- Burzynski SR, Burzynski GS, Janicki TJ (2014) Recurrent glioblastoma multiforme: A strategy for long-term survival. J Cancer Ther 5: 957-976.
- Burzynski SR, Janicki TJ, Burzynski, GS, Marszalek A, Brookman S (2014) A phase II study of antineoplastons A10 and AS2-1 in children with recurrent, refractory or progressive primary brain tumors: Final report (Protocol BT-22). J Cancer Ther 5: 977-988.
- Burzynski SR, Janicki TJ, Burzynski GS, Brookman S (2014) Preliminary findings on the use of targeted therapy with pazopanib and other agents in combination with sodium phenylbutyrate in the treatment of glioblastoma multiforme. J Cancer Ther 5: 1423-1437.
- Burzynski GS, Janicki TJ, Marszalek A (2014) Long-term survival (>20 years) of a child with brainstem glioma treated with antineoplastons A10 and AS2-1: A case report. Neuro Oncol 11: 16.
- Burzynski SR, Janicki TJ, Burzynski GS, Marszalek A (2014) The response and survival of children with recurrent diffuse intrinsic pontine glioma based on phase II study of antineoplastons A10 and AS2-1 in patients with brainstem glioma. Childs Nerv Syst 30: 2051-2061.
- Burzynski SR, Burzynski G, Janicki J, Marszalek A (2015) Complete response and Long-term survival (>20 years) of a child with tectal glioma: A case report. Pediatr Neurosurg 50: 99-103.
- Burzynski SR, Janicki TJ, Burzynski G (2015) A phase II study of Antineoplastons A10 and AS2-1 injections in adult patients with recurrent anaplastic astrocytoma: Final report (Protocol BT-15). Cancer Clin Oncol 442: 13-23.
- Burzynski SR, Janicki T, Burzynski G (2015) A phase II study of Antineoplastons A10 and AS2-1 in adult patients with primary brain tumors: Final report (Protocol BT-09), J Cancer Ther 6: 1063-1074.
- Burzynski SR, Janicki TJ, Burzynski GS, Marszalek A (2015) A Phase II Study of Antineoplastons A10 and AS2-1 in adult patients with newly-diagnosed anaplastic astrocytoma: Final report (Protocol BT-08). Cancer Clin Oncol 4: 28-38.
- Burzynski SR, Burzynski GS, Marszalek A, Janicki J, Martinez-Canca J (2015) Long-term survival (over 20 years), complete response and normal childhood development in medulloblastoma treated with Antineoplastons A10 and AS2-1. J Neurol Stroke 2: 00054.
- Burzynski SR, Burzynski GS, Marszalek A, Janicki TJ, Martinez-Canca JF (2015) Long-term survival over 21 years and pathologically confirmed complete response in pediatric anaplastic astrocytoma: A case report. J Neurol Stroke 2: 00072.
- Burzynski SR, Burzynski GS, Brookman S (2015) A case of sustained objective response of recurrent/progressive diffuse intrinsic pontine glioma with phenylbutyrate and targeted agents. J Cancer Ther 6: 40-44.
- Burzynski SR, Janicki, T, Burzynski G, Marszalek A (2015) A phase II study of antineoplastons A10 and AS2-1 in patients with brainstem gliomas: The report on non-diffuse intrinsic pontine glioma (Protocol BT-11). J Cancer Ther 6: 334-344.
- Burzynski SR, Janicki TJ, Burzynski GS (2016) Primary CNS tumors and leptomeningeal, disseminated and/or multicentric disease in children treated in Phase II studies with Antineoplastons A10 and AS2-1. Cancer Clin Oncol 5: 38-48.
- Burzynski SR, Janicki TJ, Burzynski GS (2016) A phase II study of antineoplastons A10 and AS2-1 in children with low-grade astrocytomas: Final report (Protocol BT-13). J Cancer Ther 7: 837-850.
- Burzynski SR, Janicki TJ, Burzynski GS (2017) Antineoplastons A10 and AS2-1 in the treatment of children with optic pathway glioma: Final report for protocol BT-23. Cancer Clin Oncol 6: 25-35.
- Burzynski SR, Janicki TJ, Burzynski GS, Marszalek A (2017) A phase II study of Antineoplastons A10 and AS2-1 in children with brain tumors: Final report (Protocol BT-10). J Cancer Ther 8: 173-187.
- Burzynski SR, Janicki T, Beenken S (2019) Treatment of recurrent glioblastoma multiforme (rGBM) with Antineoplaston AS2-1 in combination with targeted therapy. Cancer Clin Oncol 8: 1-15.
- Burzynski SR, Janicki T, Burzynski GS, Beenken S (2021) Long-term survival (27.7 years) following IV Antineoplaston Therapy (ANP) in a 36-year-old-female with a progressive diffuse intrinsic pontine glioma (DIPG). Int J Radiol Imaging Technol 7: 073-078.
- Burzynski SR, Burzynski GS, Janicki T, Beenken S (2021) Long-term survival (23 years) in a 26-year-old male after Antineoplaston therapy for a progressive, diffuse intrinsic pontine glioma: A case report. Int J Brain Disord Treat 6: 038-044.
- Burzynski SR, Janicki T, Burzynski GS, Beenken S (2021) Resolution of clinical signs, a complete response, and long-term survival (>23 Years) in a 3 and ½ month female with a newly diagnosed diffuse intrinsic pontine glioma treated with antineoplastons. Biomed Res Clin Prac 6: 1-6.
- Burzynski SR, Janicki T, Burzynski GS, Beenken S (2022) A 25-year-female with diffuse intrinsic pontine glioma surviving for more than nine years following treatment with antineoplastons. Int J Clin Oncol Cancer Res 7: 1-7.
- Burzynski SR, Janicki T, Burzynski GS, Beenken S (2021) diffuse intrinsic pontine glioma in an 11-year-old female treated with antineoplastons: Complete response and > 25-year survival. Ped Neonatal Med. In Press.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Forensic Research and Criminal Investigation (ISSN: 2640-0846)
- International Journal of Anaesthesia and Research (ISSN:2641-399X)
- Journal of Immunology Research and Therapy (ISSN:2472-727X)
- International Journal of Clinical Case Studies and Reports (ISSN:2641-5771)
- Stem Cell Research and Therapeutics (ISSN:2474-4646)
- Journal of Cell Signaling & Damage-Associated Molecular Patterns
- Journal of Alcoholism Clinical Research




