We propose a novel approach to develop, validate and demonstrate new electrochemical methods and corresponding devices for global analysis of the redox state of biological systems. The study included methods and associated devices developed at three levels of complexity: i) modified electrodes for electrochemical analysis of individual biological redox species, ii) an electrode array for simultaneous analysis of complex compositions of biological redox substances, and iii) a sophisticated electrode measuring total response from all biological redox substances simultaneously. The devices have been extensively characterized for their interaction with biological cells and tissues via thorough experiments. In the second part of the research, the technology has been further matured for point of care (POC) use by development of i) a self-powered bio-sensing system operated as a bio-fuel cell and measurements taken without any external power source, and ii) a sense-and-treat (theranostic) system - for automatic drug release on-demand in response to the measured redox state. Bio-sensing has been achieved using bio-catalytic cascades performing logic operations (bio-molecular information processing - “bio-computing”). The results allow real-time monitoring of physiological responses to oxidative stress, particularly those caused by chem/bio/rad (CBR) exposure which is highly important for forensic science applications, particularly emphasizing the role of oxidative stress in dating injuries or to support a forensic diagnosis.
GENERAL INTRODUCTION
Exposure to chem/bio/rad (CBR) agents leads to oxidative stress (OS), characterized by elevated levels of reactive oxygen species (ROS) and reactive nitrogen species (RNS) resulting in cell dysfunction, cell apoptosis, and tissue necrosis. The analysis of the oxidative stress in a human body is highly important for forensic science applications [1,2]. ROS stimulated oxidative damage represents a cardio toxicity factor, used in the forensic diagnosis of sudden cardiac death [3]. Furthermore, oxidative injury induced by ROS and RNS could be an indicator of carbon monoxide poisoning [4]. Measurement and real-time monitoring of oxidative species produced under OS are difficult and in general impossible - or even meaningless. This is because the extremely active radical species (ROS and RNS) appear at very low and unsteady concentrations and the damage produced by them to biological tissues and physiological processes can be hardly estimated based on direct measurements of these labile species. Instead of direct measurements of the radical species, we propose to measure much more stable biological redox (reduced) species appearing at concentrations related to the physiological response to the primary oxidative species. The research is aimed at the analysis of the global response of biological systems studied on model systems. The novelty and importance of the proposed research is in the measurement of key redox species in a standardized bio-sensing platform. The developed approach allows analysis of individual biological redox species and, more importantly, an integrated response to all species. Additional features of the approach allow autonomous operation of the sensing device without any external power source (self-powered operation) and integration of sensing and actuation (theranostic) when a drug release can follow the measured redox response. The proposed methods and corresponding devices are based on novel biotechnological methods (including bio-computing) [5] and bioelectronics and Nano-technological advance [6].
MOTIVATION AND GOAL
The work has been motivated by the need for simple and effective analysis of the global redox state of biological systems in response to CBR agents. The goals of the research included standardized bio-sensing of various (different) biological redox species. The selection of redox biomarkers relevant to the analysis of oxidative stress has been performed upon a comprehensive literature survey to identify physiological and pathological levels of known redox biomarkers (or analytes) of oxidative stress. Depending on the specific needs, the analysis has been performed separately for each analyzes at different electrodes in an array assembly or simultaneously for all analytics (integrated response) at a single electrode. Additional options included: a self-powered bio-sensing system and sense-and-treat bio-sensing-actuating system providing autonomous operation and on-demand drug release.
GENERAL DESIGN OF THE BIO-SENSING ELECTRODES
The bio-sensing devices have been developed on a standard platform for all measurable redox substances. This approach utilizes enzyme-functionalized nano-particles (NPs) assembled on grapheme-modified electrodes. The bio-sensing electrodes were prepared from carbon fiber paper (Toray paper) functionalized with in situ generated graphene nano-sheets pilled out by electrochemical treatment [7]. Hemin used as an electrochemical catalyst for reduction of bio-catalytically produced H2O2 was adsorbed on the graphene nano-sheets (strong adsorption based on p-p stacking). SiO2-nanoparticles (NPs) were solemnized with amino-silane to introduce surface amino groups, and modified with enzymes via carbodimide coupling. The enzyme-functionalized NPs were covalently bound to the electrode surface via the carboxylic groups of them in. The bio-catalytic electrode oxidized the biological redox species producing in a concomitant reaction H2O2 which was electro catalytically reduced at the hem in-modified surface, thus yielding anodic current (Figure 1).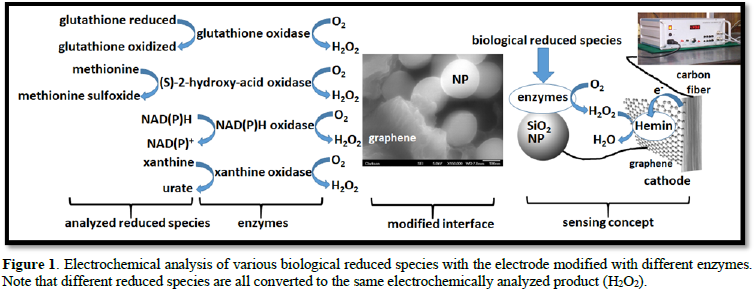
SENSING INDIVIDUAL REDOX SPECIES AT THE MODIFIED ELECTRODE
Depending on specific redox species, different enzymes (all oxidases producing H2O2) were bound to the NPs and placed at the electrode surface (Figure 1). While in the previous research only glucose oxidase and lactate oxidase were utilized, new enzymes for analysis of different redox species were tested and the system composition and operation were optimized. Note that in this experimental part of the work only one kind of redox species per electrode was analyzed.
MULTI-ANALYTE SENSING USING AN ELECTRODE ARRAY
Detection and quantification of a single biomarker in biological samples may not provide a complete physiological scenario of the redox status. For this purpose, a multi-analyte sensing platform is needed - one that can simultaneously detect several biomarkers and correlate the pathological condition to their respective disease or syndrome. An electrode array composed of many conducting areas (electrodes) was developed for analysis of complex combinations of various biological redox (reduced) species. Note that each separate electrode in the array was modified with one enzyme and used for analysis of one kind of analyze species (Figure 2).
MULTI-ANALYTE INTEGRATED SENSING USING A SINGLE ELECTRODE
In this part of the research, we modified one sensing electrode with multiple bio-catalytic enzymes (Figure 1) which produced a total response to all present biological redox species. Some redox species cannot be converted in one bio-catalytic step to H2O2 simply because of absence of appropriate enzymes. In this case, we assembled complex multi-enzyme bio-catalytic reaction cascades, resulting in formation of H2O2 to be in line with other reactions. Similar cascades have been developed by us recently [8].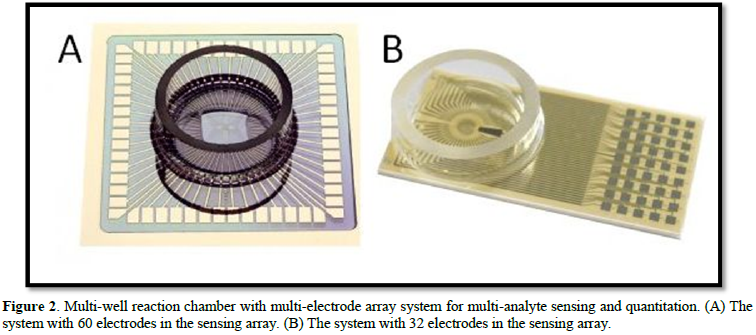
The bio-catalytic cascades performed Boolean logic operations on the analytic inputs, which were carefully planned and “programmed” by the enzyme compositions (Figure 3). This experimental approach did not provide information about concentration of individual species, but it generated an integrated response to the global concentration of the various re -dox species and its change with respect to the control.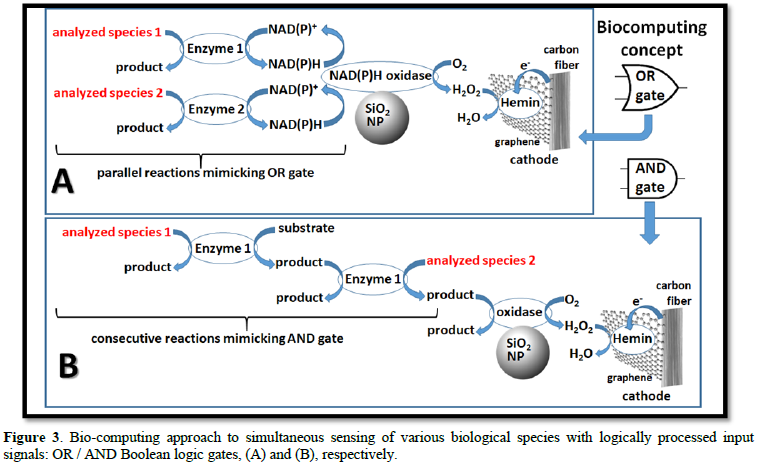
SELF-POWERED BIO-SENSING OF TOTAL COMPOSITION OF THE BIOLOGICAL REDOX SPECIES
Combining the sensing electrode with another electrode (glucose-powered anode), we assembled a bio-fuel cell which operated as a self-powered biosensor. This system does not require any external power source, thus allowing its miniaturization and wearable design. This is a critical feature towards the development of a truly POC sensor system in the future. The self-power biosensing concept was extensively tested and demonstrated via electrochemical analysis as well as testing with biological matrices.
SENSE-AND-TREAT AUTONOMOUS SYSTEM
When the sensing system operates in the self-powered mode, it can be used for on-demand drug release to alleviate the conditions that cause OS. An additional alginate-modified electrode with entrapped drug molecules in the polymeric film was added to the system (Figure 4). An electronic switch regulates the connections between the three electrodes. When the current produced by the anode-cathode pair is “normal” (physiological concentrations of the redox species) the anode and cathode are connected and the current is measured as the sensing signal. When the current changes from the “normal” (under oxidative stress) the switch connects the anode and drug-releasing alginate electrode resulting in the dissolution of the polymer film and drug release [9], thus mitigating OS conditions.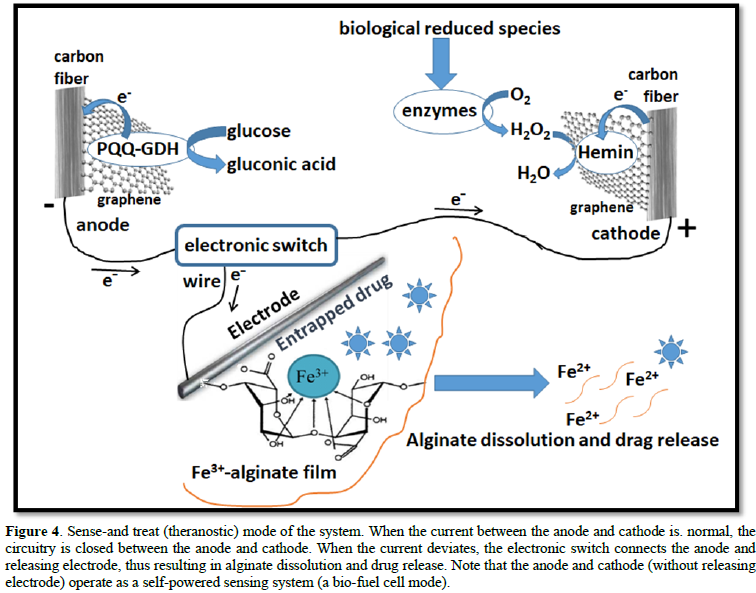
CONCLUSIONS AND PERSPECTIVES
The developed approach allows electrochemical analysis of global physiological effect of oxidative stress. The perspectives are very broad and can greatly contribute to implantable [6] and wearable [10] bioelectronics and various forensic applications [1-4].









