Mini-Review
An Insight into FDA Recall Notification of Ranitidine: A Mini Review
6218
Views & Citations5218
Likes & Shares
Ranitidine is a potent H2 receptor antagonist which is being widely prescribed globally for peptic ulcer, gastro-oesophageal reflux disease and Zollinger- Ellison syndrome. Ranitidine was considered as a promising drug with higher safety profile with impeccable tolerability, only until the recall notification announced by USFDA. USFDA recalled ranitidine in the year 2019 due the presence of N- nitroso dimethylamine (NDMA). NDMA is a potent human carcinogen which is capable of imparting cancer upon chronic therapy even at minute doses. Researchers have proved that this impurity is present in ranitidine in quantities beyond the allotted range. Zollinger- Ellison syndrome and few other diseases demands chronic therapy of H2 antagonist of which ranitidine was considered to be the safest. Such chronic therapies may lead to NDMA induced hepatotoxicity and rare chance of culminating in carcinogenesis. It is of paramount importance that the healthcare professionals should be aware of the recent updates of FDA including recalls and drug approvals and thereby result in quality prescriptions. This will indirectly benefit the public health and healthcare system.
Keywords: USFDA, Recall notification, Ranitidine, NDMA, Carcinogenesis, Awareness
INTRODUCTION
A potent histamine H2 receptor antagonist, Ranitidine which is already a well-known drug for the treatment of gastric acid secretion, peptic ulcer disease, Gastro- Oesophageal Reflux Disease and Zollinger- Ellison syndrome [1]. It has promising tolerability and efficacy profiles and mostly considered as first line drug for the conditions in both oral and parenteral routes [2]. The adverse effects of ranitidine are also reported very rarely worldwide like vitamin B12 deficiency, headache, diarrhea and constipation. The drug is also very safe, effective and tolerable in pediatric patients from 1 month to 16 years of age [3].
In the year 2019, September the drug Ranitidine with brand name Zantac was recalled by United States Food and Drug Administration (USFDA) and was investigated for the impurity. This was a nitrosamine impurity named N- nitroso dimethylamine (NDMA) which was found in the recalled drugs. Laboratory results showed that this impurity was higher than that of allowed quantity. NDMA is a human carcinogen which can cause cancer. The environmental contaminant NDMA is mainly found in water bodies and food such as vegetables, dairy products and meat [4]. USFDA recall for a marketed drug is quite usual incidents but the recall of this safest drug was unpredicted and the drugs was taken back from the market as soon as possible.
Within a week after the USFDA announcement about the NDMA impurity in ranitidine, Health Canada stopped the distribution of the drugs followed by European nations such as Germany, France, Italy and Switzerland. Some countries like Taiwan also warned the pharmacies that legal actions will be taken upon further selling. Pakistan also banned all the manufacturing and distribution of the drug [5]. In India, Drug Controller General of India (DCGI) alerted about the USFDA recall and directed the manufacturers to ensure patient safety. DCGI announced that all patients on ranitidine should shift or take the consideration of another group of acid blockers after getting an opinion from their healthcare professionals [6].
N- NITROSO DIMETHYLAMINE (NDMA)
NDMA is also known as dimethyl nitrosamine (DMN), which is an organic compound and is semi- volatile. It is an environment-based contaminant mainly found in water and food which have no taste and odor. It is soluble in water and is yellow in color. It is a chemical compound usually formed as a by- product for the industrial processes [7]. NDMA is highly toxic human carcinogen predominantly to the liver [8]. And is registered in (United States Environmental Protection Agency) USEPA’s Contaminant Candidate List 3 [9]. It can cause liver fibrosis when administered in high doses due to its potent hepatotoxic activity. It is used for the animal studies to induce liver injury in rats [10]. Also, for in vivo studies of cancer it is used for the induction of tumor in rats at chronic exposure [11].
It is known that these impurities can be formed during the production under certain conditions and when certain solvents, reagents, and other raw materials are used. In addition, it is possible that impurities were present in some ranitidine because manufacturers had inadvertently used contaminated equipment or reagents in the manufacturing process.
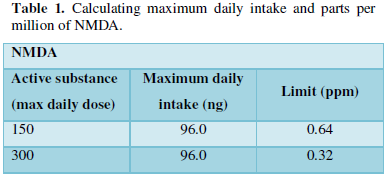
Ranitidine medications are generally available in two different strengths as 150 mg and 300 mg tablets. The maximum daily intake of NDMA is 96.0 nanograms. The limits are based on the maximum daily intake for each impurity derived from animal studies. Dividing these by the maximum daily dose for each active substance gives the limit in parts per million. For the available strengths 150 mg and 300 mg, the limits measured were 0.64 ppm and 0.32 ppm (Table 1) respectively which exceeds 0.16 ppm, the currently agreed threshold for NDMA in ranitidine hydrochloride [12]. Ranitidine is expected to have very low concentration of NDMA formation but it can be maximized in some conditions which should be avoided [13].

AWARENESS IN HEALTHCARE PROFESSIONALS
The USFDA alerted the healthcare professionals like physicians and pharmacists by publishing the news by the BMJ publishing group about the contamination of ranitidine with the carcinogen NDMA. Also, US’ National Drug Code identified the batches from Glenmark Pharmaceuticals which was recalled and stop the supply of ranitidine till the investigation. UK and India also recalled many lots of products of ranitidine by the Glenmark Pharmaceuticals. And it was requested to switch to clinical alternatives for the treatment of the patients [14].
A descriptive, cross sectional study in Chennai, Tamil Nadu, India carried out for 2 months in 2019 for the ranitidine recall awareness among 311 physicians and pharmacists. The study was done in both rural and urban areas in which most of the participants were aware of the ranitidine recall notification from FDA and the impurity identified NDMA.is classified as probable human carcinogen. When it comes to source of impurity, only 14% of the Physicians among the study population were aware of the source of impurity. 90% of the Pharmacists as they work in the core area, they were aware of the source of the impurity identified. Less than 5% of the study participants both physician and pharmacists were aware of the currently agreed threshold of the impurity and the maximum daily intake of the same. Only 58% of the Pharmacists and 5% of the Physicians were able to calculate the limit of the impurity in parts per million. It was appreciable that the physicians and pharmacists who were well aware of the study content, stated advising their patients with newer H2 blockers. It was concluded that the healthcare professionals should update themselves with the latest information for the safety of patients [15].
As for the awareness among healthcare professionals, USFDA have constantly evaluated the quality and safety of the drugs either old or new and gain the knowledge about the drugs to minimize the unknown risk to the patients by various testing methods which is now a days have become more sophisticated and sensitive [16].
CONCLUSION
The USFDA is taking all measures to ensure safety, efficacy, tolerability and adverse effects of the drugs. Healthcare professionals also need to report the adverse drug reactions mainly in the over-the-counter (OTC) drugs to the pharmacovigilance department or to the FDA MedWatch program. Government bodies like World Health Organization (WHO), USFDA, or Indian Council of Medical Research (ICMR) can conduct some seminar or conferences for the healthcare professionals so that they will get updated about the new drugs in the market or the new adverse effects by the post marketing surveillance and about the recalled and banned drugs which will improve their prescription quality. This way the public health will be benefitted and also the healthcare professionals will be aware of the latest information by the regulatory.
- Dawson J, Richards DA, Stables R, Dixon GT, Cockel R (1983) Ranitidine - pharmacology and clinical use. J Clin Hosp Pharm 8(1): 1-13.
- Grant SM, Langtry HD, Brogden RN (1989) Ranitidine. Drugs 37: 801-870.
- USFDA (2014) Zantac drug insert. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/018703s068,019675s035,020251s019lbl.pdf
- USFDA (2020) FDA updates and press announcements on NDMA in Zantac (ranitidine). Available online at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-and-press-announcements-ndma-zantac-ranitidine
- USFDA (2019) Zantac recall: FDA studies cancer risk in ranitidine, heartburn drugs (usatoday.com) Available online at: https://www.usatoday.com/story/news/health/2019/11/07/how-did-zantac-become-potential-cancer-risk-fda-wants-find-out/2509043001/
- USFDA (2019) Ranitidine ban: Indian drug regulator 'alerts' state authorities to ensure patient safety (businesstoday.in). Available online at: https://www.businesstoday.in/industry/pharma/story/ranitidine-ban-indian-drug-regulator-alerts-state-authorities-to-ensure-patient-safety-dcgi-232909-2019-10-01
- Deka S, Bhattacharjee A (2018) Safety of a drug beyond molecule: what we have learned from recall of valsartan. Int J Curr Med Pharm Res 4(11A): 3884-3887.
- Andrzejewski P, Kasprzyk-Hordern B, Nawrocki J (2005) The hazard of N-nitroso dimethylamine (NDMA) formation during water disinfection with strong oxidants. Desalination 176(1-3): 37-45.
- U S Environmental Protection Agency (1987) Integrated Risk Information System (IRIS), N-nitroso dimethylamine. Office of Research and Development (ORD), National Centre for Environmental Assessment. Accessed on: October 20, 2011. Available online at: https://www.epa.gov/iris/subst/0045.htm
- George J, Rao KR, Stern R, Chandrakasan G (2001) Dimethyl nitrosamine-induced liver injury in rats: the early deposition of collagen. Toxicology 156(2-3): 129-138.
- Richard P, Richard G, Paul B, Paul G (1991) Dose and Time Relationships for Tumor Induction in the Liver and Oesophagus of 4080 Inbred Rats by Chronic Ingestion of N-Nitrosodiethylamine or N-Nitroso dimethylamine. Cancer Res 51: 6452-6469.
- USFDA (2019) FDA Updates and Press Announcements on Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) Method for the Determination of NDMA in Ranitidine Drug Substance and Solid Dosage Drug Product. Available online at: https://www.fda.gov/media/131868/download
- Roux JL, Gallard H, Croué JP, Papot S, Deborde M (2012) NDMA Formation by Chlorination of Ranitidine: Kinetics and Mechanism. Environ Sci Technol 46(20): 11095-11103.
- Mahase E (2019) Ranitidine: patients taking certain batches should “immediately discontinue use,” says FDA. BMJ 367: l7053.
- Begam RF, Ravikumar Y, Velmurugan R (2021) Awareness about FDA announcement on voluntary recall of ranitidine among physicians and pharmacists in and around Chennai, India: A cross-sectional study. Future J Pharm Sci 7(1): 112.
- Woodcock J (2019) Statement on new testing results, including low levels of impurities in ranitidine drugs. FDA STATEMENT. Available online at: https://www.fda.gov/news-events/press-announcements/statement-new-testing-results-including-low-levels-impurities-ranitidine-drugs


