Research Article
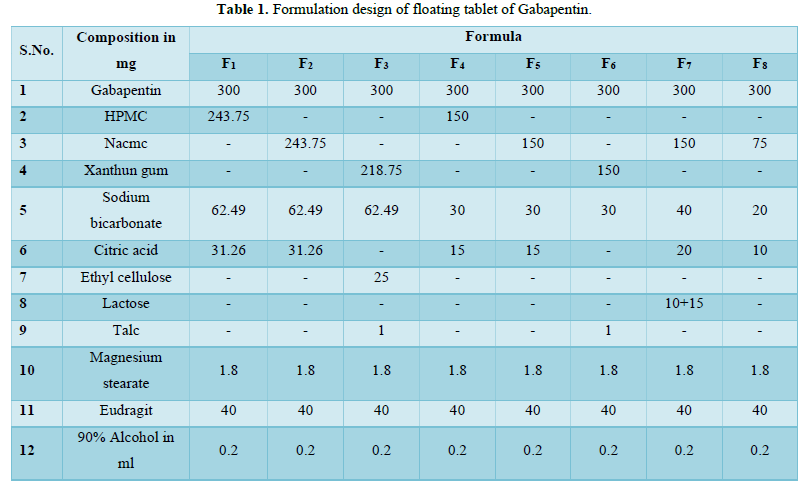
Formulation of Gastro Retentive Drug Delivery System & it’s In vitro Evaluation
2977
Views & Citations1977
Likes & Shares
This work was carried out to prepare and evaluate the floating oral sustained release tablet drug delivery system of Gabapentin by using HPMC K15, Nacmc, Xanthun gum, Sodium bicarbonate, Citric acid. Lactose, Ethyl cellulose as polymers, which are primarily absorbed from the stomach by increasing the GRT of the drug. NaHCo3 and citric acid are used as effervescent agents for floating the drug. Lactose is used to modify the release of some formulations. Ethyl cellulose is given in some formulations to retard the disintegration and also dissolution. The study also includes various evaluation studies of Gabapentin tablets and the effect of processing variables on them. A possible interaction between drug and polymers are also investigated by FTIR and DSC studies.
Keywords: Gabapentin, NaHCo3, FTIR, Gastro retentive floating tablet, Gastric emptying time
INTRODUCTION
Gastro retentive drug delivery systems such as floating tablets are especially effective in the delivery of weakly acidic drugs which are absorbed mainly in the stomach such as gabapentin. The gastro-retentive floating tablet will alter beneficially the absorption profile of the active agent, thus enhancing its bioavailability. Drugs that have poor bioavailability because of their limited absorption to the intestinal tract can also be delivered efficiently by floating in the stomach thereafter maximizing their absorption and improving the bioavailability [1,2].
The design of Floating Drug Delivery Tablets (FDDS) should be primarily aimed to achieve more predictable and increased bioavailability. Now a day most pharmaceutical scientist is involved in developing the ideal FDDS. This ideal system should have the advantage of a single dose for the whole duration of treatment and it should deliver the active drug directly at the specific site. Scientists have succeeded to develop a system and it encourages scientists to develop control release tablets [1-3].
Rationale
To modify the molecular structure or physiological parameters inherent in a selected route of administration.
To alter the pharmacokinetics and pharmacodynamics of pharmacologically active moieties by using novel drug delivery tablets.
So, the present project has been undertaken to design and evaluate of floating oral sustained release tablet drug delivery system of gabapentin by using HPMC K15, Nacmc, Xanthun gum, Sodium bicarbonate, Citric acid, Lactose, Ethyl cellulose etc. [4,5].
MATERIALS AND METHOD
Materials
All the chemicals used were of pharmacopeia grade. Analytical Electronic Balance- Ohaus, Adventurer, India, Hot Air Oven-NSW, India, Rotary Punch Machine (8 Stations) -CIP, Lab Press. India., pH Meter Apparatus- ELICO India, Model: -LI 120, 8 Stage Dissolution Apparatus- Electrolab, Model: -TDT-08 L, USP, HPLC- Shimadzu, Model: -LC 20AT Prominence, FTIR- Spectrophotometer: NEXUS 870 FTIR [Thermo Nicolet], Differential Scanning Calorimeter Perkin Elmer [Pyris Diamond DSC] USA.
Method
Method of preparation of floating tablet of Gabapentin
Gabapentin floating tablets were prepared by the wet granulation method. The drug was mixed with the polymer according to Table 1 in a mortar and pastel and it was triturated properly to form a uniform distribution of drug and polymer. An alcoholic solution of eudragit of the tabulated amount was added as a binder, to make granules after adding sodium bicarbonate as effervescent. The wetted mass was then passed through sieve no. 40# and dried at 450°C in a hot air oven for 15 min. The dried granules were mixed with magnesium stearate, talc and the whole mass/granules were compressed by 10 mm flat die and punched after passing through 60# size. In some cases, citric acid was used add to increase buoyancy i.e., in formulation no. F1, F2, F4, F5, F7 and F8.

Evaluation of Floating Tablet
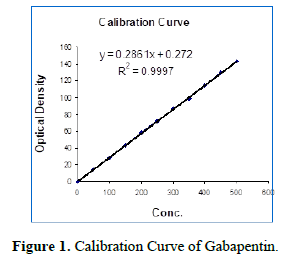
Preparation of calibration curve: Calibration curve of standard gabapentin was prepared by using the HPLC method according to USP 2008.
Preparation of mobile phase for HPLC by dissolving 1.2 gm of KH2PO4 in double-distilled water in 1 lt volume-metric flask and 60 ml of acetonitrile and the volume was made up to 1 lt With Water and sonicated for 15 min and pH adjusted to 6.9 by adding 5N KOH solution after pH adjustment degassed by filtration with 0.45 µm membrane filters.
Preparation of drug solution: A stock solution of gabapentin of conc. 1000 mcg/ml in 0.1N HCl was prepared. The said solution was again diluted to a series of concentrations in ascending order i.e., 50, 100, 150, 200, 250, 300, 350, 400, 450, and 500 µg/ml in 0.1N HCl. 20 µl of the above solution was injected in to HPLC with a flow rate of 1.2 ml/min and detected under UV detector at 210 nm. The regression coefficient was also shown in Figure 1 [6,7].

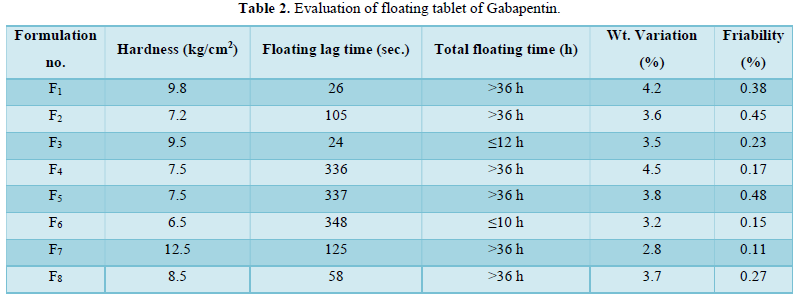
Hardness test: For this study 10 tablets of each formulation were taken and using Monsanto tester the tablet crushing strength (pressure) was done for an individual tablet of each formulation.
Floating time test: For invitro evaluation of floating time behavior studies, 10 tablets were taken for this experiment. each tablet of all formulations was dropped into 250 ml beakers containing 0.1N HCl. The floating lag time and the total gross floating time were noted by the stopwatch. The average floating lag time of all formulations was calculated.
Weight variation study: For this study 10 tablets of each formulation were weighed collectively and individually. From the collective weight, the average weight per tablet is calculated. the weights of individual tablets were then compared with avg. wt to ascertain whether the same is within the permissible limit or not.
Friability test: It is the subjective combined effect of abrasion and shock by utilizing a plastic chamber that revolves at 25 RPM dropping the 20 tablets at a distance of 6 inches with each revolution (according to USP). Normally a pre-weighed tablet sample is placed in an originator which is then rotted for 100 revolutions. The tablet was then reweighted and compared with the initial weight. the combined results of the above-mentioned parameters are shown in Table 2.

Evaluation of floating tablet through HPLC
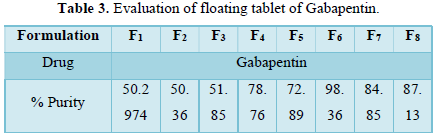
Drug content (assay): 10 tablets were taken in each experiment. after triturating 10 per weighted tablet, 50 mg equivalent weight of drug was taken in a 100 ml of volumetric flask diluted to 0.1 HCl and kept for 24 h for drug dissolution in the medium. Then the resulting mixture was sonicated for 30 min and the volume was made up to 100 ml with 0.1N HCl, Then the sample was injected into the HPLC system after filtration and detected by UV-Detector at 210 nm. The results are shown in Table 3.

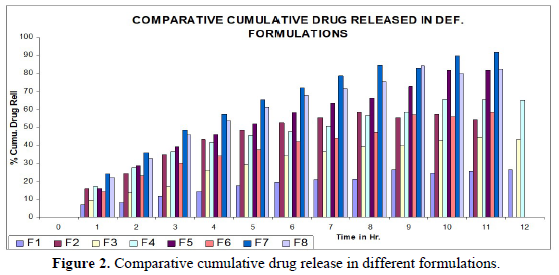
Invitro drug release study: Invitro release of Gabapentin tablets first pre-weighed tablets of different formulations were stirred in 900 ml dissolution medium at 100 RPM. A sampling of 10 ml was withdrawn at a predetermined (30 min) time interval and replenished with the same volume of fresh medium. These formulations were perfumed in 0.1N HCl at (37 ± 0.5) ºC using USP slandered basket-type dissolution test apparatus (ELECTROLAB Disso 2000). The 20 µl sample was analyzed by HPLC at 210 nm using a UV detector at the run time of 8 min (Figure 2).
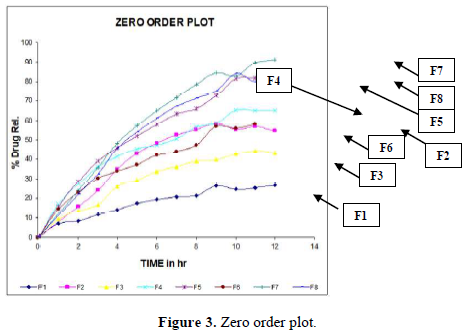
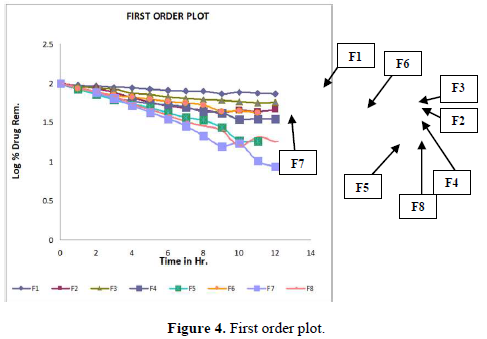
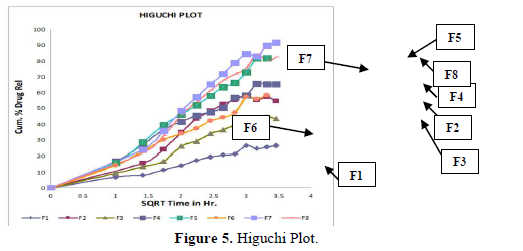
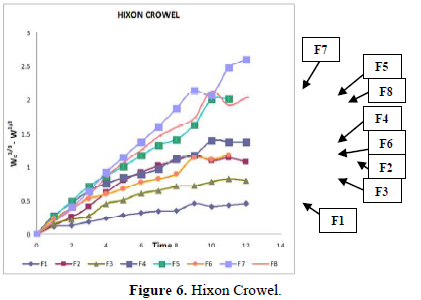
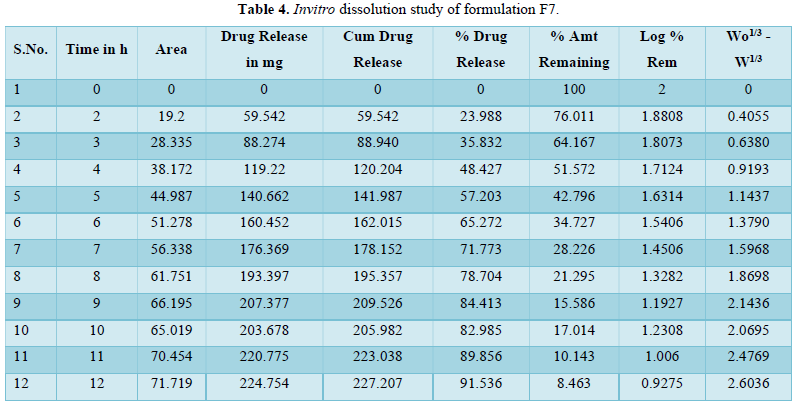
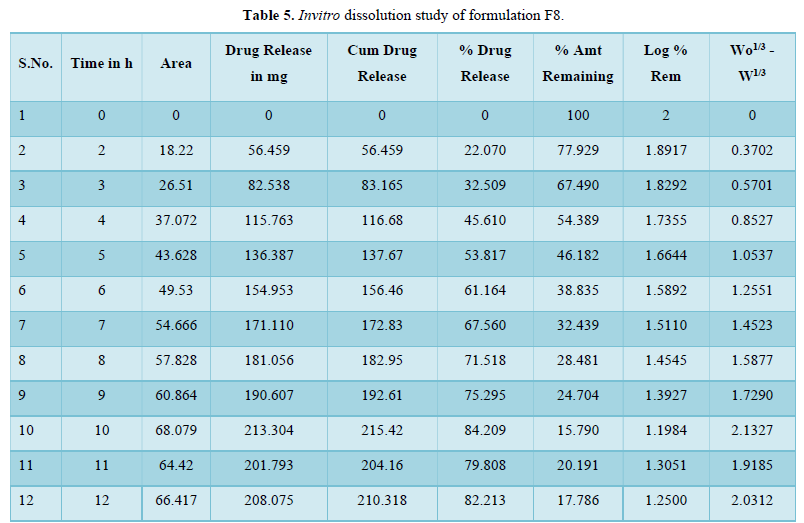
Evaluation of drug release mechanism by various kinetic models like zero order, first order, Higuchi equation and Hixon Crowell equation from F1 to F8 in Figures 2-6 and Tables 4 & 5. From this table, we got that in all the formulations the correlation coefficient values of the Higuchi Square Root equation were high and the R2 value of the Hixon Crowell Cube Root equation was second highest. So, we confirmed that all formulations followed diffusion control released of gabapentin or are revealed that diffusion was the predominant process of release in all the tables. An R2 value of the Higuchi equation ranges from 0.9390 to 0.9916. In all formulations, the diffusion control release was followed by surface Erosion Kinetics.







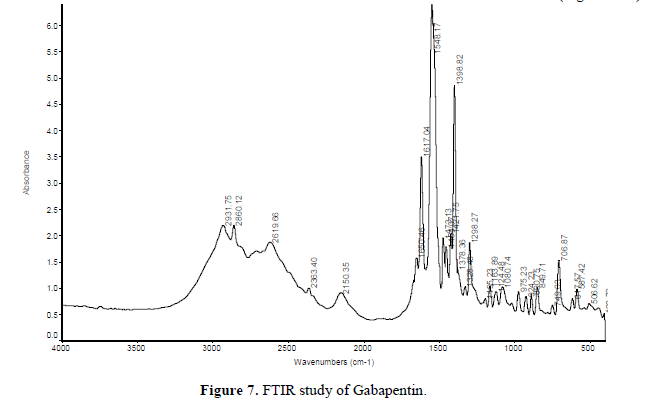
FTIR study
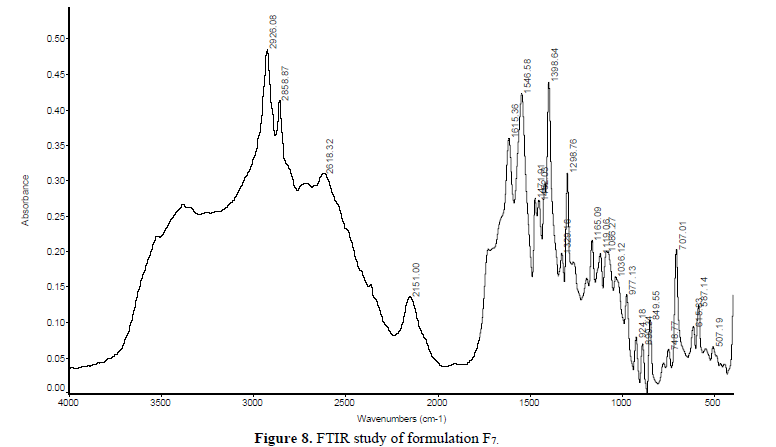
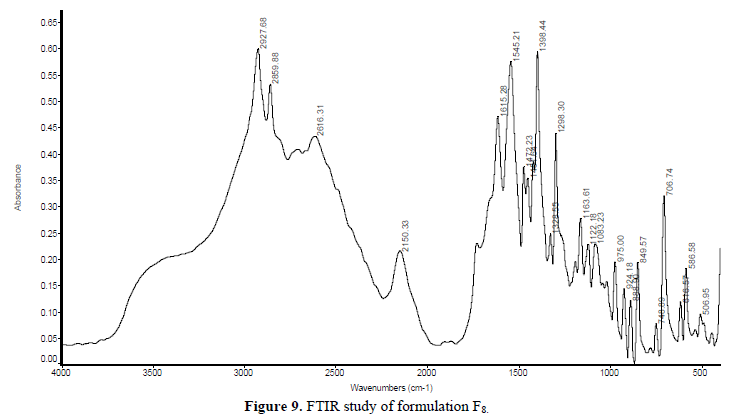
FTIR spectra of a pure drug (Gabapentin), blank polymer and drug-loaded tablets were obtained separately at room temperature in Kbr pellets using NEXUS 870. FTIR [THERMO NICOLET] in Central Research Lab., IIT KHARAGPUR between the wavenumber range of 500 to 4000 cm-1 and resolution about 2 cm-1 (Figures 7-9).



DSC study
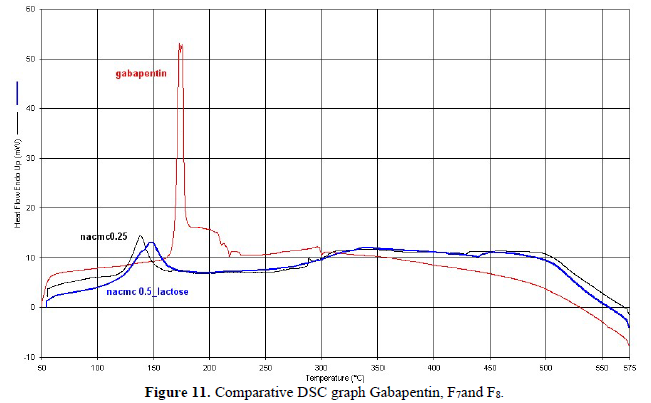
DSC-curve of Gabapentin and the tablet formulation of F7 and F8 were recorded with a differential scanning calorimeter (Perkin Elmer) in Central Research Lab., IIT KHARAGPUR from 50 To 575oC at a heating rate of 5oC/min. The instrument was calibrated using indium as standard. 4.84 mg of sample were placed in aluminum pans and sealed. Reproducibility was checked by running the sample in Figures 10,11.



RESULTS AND DISCUSSION
Drug content of various formulations: The percentage drugs content of gabapentin in the floating tablet were shown in Table 6. The formulation F6 showed the highest drug retained value i.e., 98.36% and F1 showed the lowest drug retained value i.e., 50.29%.
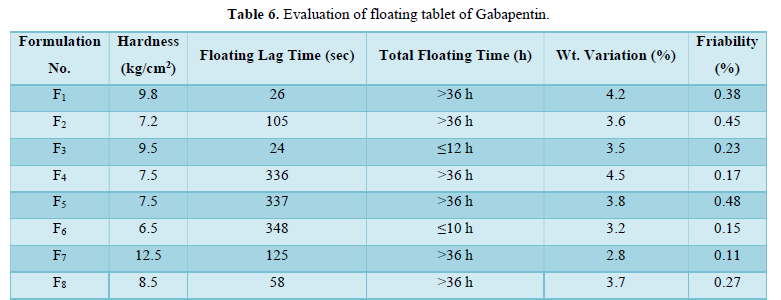

Floating ability/ Buoyancy of tablet: The Gabapentin floating tablet was shown to give good floating ability. each tablet shows 36 h floating efficiency except F3 and F6 because the formulation F3 and F6 contains Xanthun gum which was shown 12 h floating action only due to its swelling nature.
Effect of sodium carbonate on floating: The floating lag time of each formulation decreased with an increase in the concentration of sodium carbonate and the total floating time increased with an increase in conc. of NaHCo3.
Effect of citric acid on floating: Similarly, the more amount of citric acid increases the floating time and decreases floating lag time.
Invitro release behavior of drug: In this experiment different formulations F1, F3 were prepared, T50 values lie beyond 12 h. In F2 formulation the T50 is about 6-7 h and the highest drug release is 57% at 11 h and it was decreasing its release profile at 12 h i.e., 54%. Similarly, like that F4 at 11 h, it is 56.3% but at 12 h it is 65%. But in the formulation F1, F3, F4 the drug release profile is very slow i.e., at 12 h it is only 26% in F1, 44% in F3, and F4 it is 65% at 12 h. In this above formulation F1, F4 have polymer PMCE15LV at the different ratios with a drug that has a low release profile of drug but in formulation F7 and F8, the drug release profile is 91.5% in 12 h and F8 is 82% at 21 h and obeying first-order release kinetics with correlation factor 0.985021 and 0.9683055 respectively giving sustained action. F7 and F8 containing the polymer Na CMCI the ratio with the drug is 1:0.5 and 1:0.25. F8 contains less polymer than F7 and gives more sustaining action because F7 contains lactose which increases the aqueous entrapment to the formulation. But the F7 formulation shows greater drug release in 12 h i.e., 91%. So here it was concluded that the rate of drug release from all the formulations was sustained as the increase in the polymer conc. The drug release kinetics in all formulation was different due to their differences in composition. The drug release was more sustained in F1 whereas it was less sustained in F7. The reason behind the differentiation in drug release kinetics was based upon the nature of polymers and the drug-polymer ratio. From the linearity data of the Higuchi equation mentioned in the, it was concluded that all formulations are obeying Higuchi equation i.e., in all formulations. The drug was released in the diffusion process.
IR spectrum study: The polymer, drug, and formulation structure were confirmed by IR spectroscopy. The IR spectra of Nacmc, it is evident that it shows a broad observation band at 3408.21 cm-1, due to the stretching frequency of the -OH group. The band at 2922.26 cm-1 is due to C-H stretching vibration. The presence of a strong observation, band at 1598.04 cm-1 confirms the presence of the COO group. The band around 1418.13 cm-1 and 1326.89 cm-1 are assigned to -CH2 scissoring and -OH bending vibration, respectively. The band at 1060.44 cm-1 is due to >CH-OCH2 stretching. The IR spectra of Gabapentin show an abroad observation band at 2931.75 cm-1 due to the C-H stretching of Cyclohexane. The presence of a strong observation band at 1459.07 cm-1 indicates C-H bending in Cyclohexane (saturated) six-membered rings. The band at 1328.48 cm-1 indicates the presence of C-O stretching for Carboxylic Acid and the band at 1421.75 cm-1 indicates the C-O-H bending. The band at 1298.27 cm-1 indicates the presence of C-N bond stretching and the band at 1650.46 cm-1 indicates the presence of N-H (scissoring) vibration. From the above-given data, it is concluded that, in the formulation F7, F8 there was no interaction between the drug and polymer.
DSC study: The present study describes the application of Differential Scanning Calorimetry (DSC) to ascertain the crystalline state of a drug with a melting point of 173.02°C after dispersion on hydrophilic carriers by either simple mixing or by fusion. Whereas in formulation F7 and F8 the melting point range was found 138.2°C to 146.31°C. The most interesting of the systems investigated, in which the drug is gradually transformed from the crystalline to the amorphous state at high temperature so it helps to increase drug solubility.
CONCLUSION
The choice of Gabapentin is done for floating tablet due to its potent antiepileptic nature especially used in the treatment of epilepsy and postoperative neuropathic pain, so also it is a weakly acidic drug having pH 6.5-7.5, bioavailability is about 60%, protein binding is >3%, the initial adult dose is about 300mg/3times/day. The important aspect of this drug is that food increases absorption by about 14%, so taking into consideration the above data, Gabapentin is considered as the suitable candidate for the floating drug delivery system especially tablets. From all the above results, we concluded that the major advantage of the system is its high floating ability and sustained release of the drug over several hours. Among all the formulations, F8 showed a better-sustained effect with an effective floating ability. So, the formulation F8 was more effective in epilepsy management owing to the better pharmacological response.
- Arora S, Ali J, Ahuja A (2005) Floating drug delivery systems: A review. AAPS PharmSciTech 6(3): E372-E390.
- Vyas SP, Khar RK (2001) Gastro retentive Systems, In Controlled Drug Delivery: Concepts and Advances. Vallabh Prakashan, Delhi. pp: 197.
- Deshpande AA, Shah NH, Rhodes CT (1997) Development of A Novel Controlled Release System for Gastric Retention. Pharmres 14: 815-819.
- Basak SC, Rahman J, Ramalingam M (2007) Design and invitro testing of a floatable gastro retentive tablet of metformin hydrochloride. Pharmazie 62(2): 145-148.
- Tokumura T, Macshida Y (2006) Preparation of amoxicillin intragastric buoyant sustained-release tablets and the dissolution characteristics. J Control Release 110(3): 581-586.
- Davis SS (2005) Formulation strategies for absorption windows. Drug Discov Today 10(4): 249-257.
- Lachman L, Lieberman HA (2013) The Theory and Practice of Industrial Pharmacy. Varghese Publishing House, 3rd pp: 430.












