2489
Views & Citations1489
Likes & Shares
- Metformin should be started at the time type 2 diabetes is diagnosed unless there are contraindications; for most patients this will be monotherapy in combination with lifestyle modifications [2].
-
If the HbA1C target is not achieved after approximately 3 months and the patient does not have atherosclerotic cardiovascular disease or chronic kidney disease (ASCVD or CKD), consider a combination of metformin and any one of the preferred six treatment options: sulfonylurea, thiazolidinedione, dipeptidyl peptidase 4 (DPP-4) inhibitor, SGLT2 inhibitor, GLP-1 receptor agonist, basal insulin, the choice of which agent to add is based on drug-specific effects and patient factors [2].
- Till date, only a few studies have been done comparing efficacy of empagliflozin versus teneligliptin as add on therapy to metformin monotherapy for treating T2DM. Further, to the best of our knowledge, no such study has been conducted in India. Hence, this study was conducted to compare the efficacy and safety of empagliflozin versus teneligliptin as add on therapy to metformin in patients of uncontrolled T2DM [2].
- In most of the comparative studies of empagliflozin with teneligliptin, the duration of study was six months or twelve months. Since, this was a time bound academic study; the participants were followed-up for six months [2].
MATERIALS AND METHODS
Study Design and Setting
The study was randomized, prospective, open label, comparative interventional study. The study was conducted in the Department of Pharmacology and the Department of Medicine at Dr. R.P.G.M.C, Kangra at Tanda which is 700 bedded multispeciality tertiary health care center situated in the foothills of Dhauladhar mountain range, at altitude of 32.0986360N and longitude of 76.3003390 E, amidst the serene Kangra valley of Himachal Pradesh in India.
IEC approval vides letter no. IEC/139/2019: dated 17/12/2019.
CTRI registration no. (REF/2020/03/032516) Trial completed on 24/10/2021.
Study population:
The study population was the consenting adult patients of T2DM of different socio-economic strata, from the Kangra and adjoining 5 districts of Himachal Pradesh. The patients were selected on an outpatient department basis.
Inclusion criteria:
- Willing to give written informed consent for the study.
- Adult patients of age more than 18 years of either sex.
- Ambulatory subjects who were suffering from type 2 diabetes mellitus and prescribed anti-diabetic drug at medicine OPD.
Exclusion criteria:
- Subjects age less than 18 years.
- Subjects not willing to participate.
- Coexisting cardiac, renal, liver and CNS emergency conditions.
- Any condition resulting in severe learning disability (e.g. brain injury) or unable to comprehend for other reasons.
- Acute complications of diabetes mellitus such as hyperglycemic hyperosmolar state and diabetic ketoacidosis.
- Pregnancy and lactating mothers.
- Known hypersensitivity or contraindications to study drugs.
- Patients already on study drugs.
Study duration: The study stretched over a period of one year for the enrollment of patients and follow-up was done at the end of first, third and sixth month after initiating the treatment.
Study intervention: Detailed history of the patients with T2DM was elicited, clinical examination was done and hematological and biochemical investigations were carried out.
Once diagnosed, the registered patients of T2DM were informed about the study through the patient information sheet and were allowed to understand thoroughly about the study and related aspects in their own language.
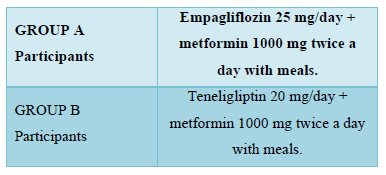
After a written informed consent, the participants were assigned to either group either A or B, based on computer generated random numbers.
receptor agonist, basal insulin, the choice of which agent to add is based on drug-specific effects and patient factors [2].
- Till date, only a few studies have been done comparing efficacy of empagliflozin versus teneligliptin as add on therapy to metformin monotherapy for treating T2DM. Further, to the best of our knowledge, no such study has been conducted in India. Hence, this study was conducted to compare the efficacy and safety of empagliflozin versus teneligliptin as add on therapy to metformin in patients of uncontrolled T2DM [2].
- In most of the comparative studies of empagliflozin with teneligliptin, the duration of study was six months or twelve months. Since, this was a time bound academic study; the participants were followed-up for six months [2].
MATERIALS AND METHODS
Study Design and Setting
The study was randomized, prospective, open label, comparative interventional study. The study was conducted in the Department of Pharmacology and the Department of Medicine at Dr. R.P.G.M.C, Kangra at Tanda which is 700 bedded multispeciality tertiary health care center situated in the foothills of Dhauladhar mountain range, at altitude of 32.0986360N and longitude of 76.3003390 E, amidst the serene Kangra valley of Himachal Pradesh in India.
IEC approval vides letter no. IEC/139/2019: dated 17/12/2019.
CTRI registration no. (REF/2020/03/032516) Trial completed on 24/10/2021.
Study population:
The study population was the consenting adult patients of T2DM of different socio-economic strata, from the Kangra and adjoining 5 districts of Himachal Pradesh. The patients were selected on an outpatient department basis.
Inclusion criteria:
- Willing to give written informed consent for the study.
- Adult patients of age more than 18 years of either sex.
- Ambulatory subjects who were suffering from type 2 diabetes mellitus and prescribed anti-diabetic drug at medicine OPD.
Exclusion criteria:
- Subjects age less than 18 years.
- Subjects not willing to participate.
- Coexisting cardiac, renal, liver and CNS emergency conditions.
- Any condition resulting in severe learning disability (e.g. brain injury) or unable to comprehend for other reasons.
- Acute complications of diabetes mellitus such as hyperglycemic hyperosmolar state and diabetic ketoacidosis.
- Pregnancy and lactating mothers.
- Known hypersensitivity or contraindications to study drugs.
- Patients already on study drugs.
Study duration: The study stretched over a period of one year for the enrollment of patients and follow-up was done at the end of first, third and sixth month after initiating the treatment.
Study intervention: Detailed history of the patients with T2DM was elicited, clinical examination was done and hematological and biochemical investigations were carried out.
Once diagnosed, the registered patients of T2DM were informed about the study through the patient information sheet and were allowed to understand thoroughly about the study and related aspects in their own language.
After a written informed consent, the participants were assigned to either group either A or B, based on computer generated random numbers.
- Before initiating the treatment, baseline blood biochemistry parameters were done.
- Patients were contacted telephonically on the next day of initiating the therapy and enquired for any adverse event.
- Patients were called for follow up at 1 month, 3 month and 6 months for therapeutic outcome and adverse event monitoring.
- HbA1c <7% was taken as adequate control of diabetes mellitus. Blood biochemical parameters were also repeated.
- Randomization was computer generated.
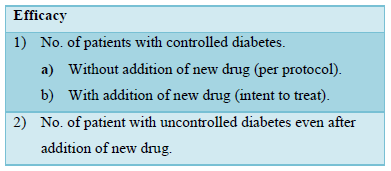
Measurement of outcome:
Statistical analysis
The data were recorded on Microsoft excel spreadsheet. Statistical analysis was done using Microsoft excel and online SPSS software. Quantitative data was presented as mean ± SD. Categorical data was presented as frequency and percentage. Student’s t-test was used for comparing continuous variables between the two groups. Chi square or Fisher’s exact test was used for comparing the qualitative data between the two groups. An intention-to-treat analysis was done to compare the data. p value < 0.05 was considered significant.
OBSERVATIONS AND RESULT
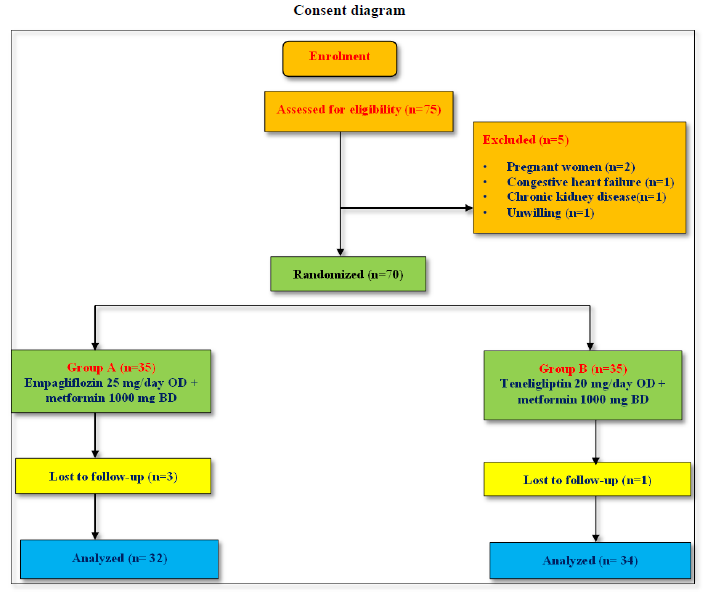
As shown in consort diagram, 75 patients were enrolled and assessed on the basis of eligibility criteria. (n) represents number of patients. 70 patients were randomized in 2 groups.5 patients were excluded on the basis of exclusion criteria. Group A (n=35) were given with empagliflozin 25 mg/day OD + metformin 1000 mg BD. Group B(n=35) were given with teneligliptin 20mg/day OD + metformin 1000 mg BD. 3 patients in Group A were lost to follow up.1 patient in group B were lost to follow up.32 patients in Group A and 34 patients in Group B were analysed.
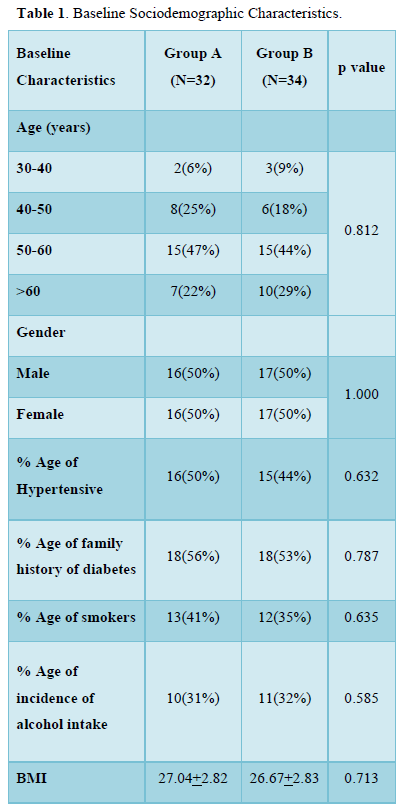
As shown in Table 1, in group A and B, majority of patients were in 50-60 years of age group. 16(50%) patients in group A and 17 (50%) in group B were males. 16 (50%) patients in group A and 17 (50%) in group B were females. 18(56%) patients in group A and 18(53%) patients in group B had family history of hypertension. 18(56%) patients in group A and 18(53%) patients in group B had family history of diabetes. 13(41%) patients in group A and 12(35%) patients in group B had history of smoking. 10(31%) patients in group A and 11(32%) patients in group B had history of alcohol intake.
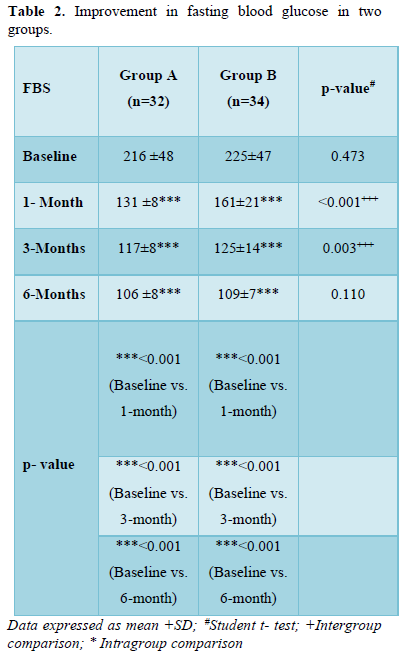
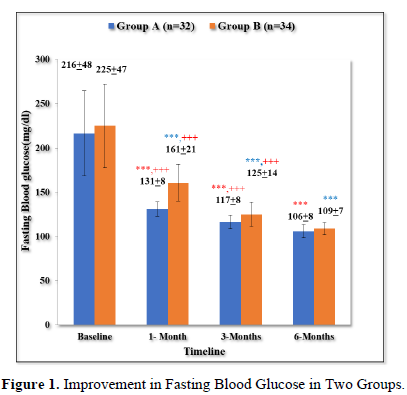
Improvement in fasting blood glucose in two groups
As shown in Table 2 and Figure 1, there was progressive significant decrease in fasting plasma glucose in both the groups over 6 months. In Group A values improved from baseline of 217+48 mg/dl to 131+8 mg/dl (p < 0.001) at 1 month; 117+8 mg/dl (p < 0.001) at 3 months; 106+8 mg/dl (p < 0.001) at 6 months.
Similarly, In Group B values improved from baseline of 225+47 mg/dl (p < 0.001) to 161 +21 mg/dl (p < 0.001) at 1 month; 125+14 mg/dl (p < 0.001) at 6 months.
Moreover, the patients in group A had statistically significant lower levels of FBS in comparison to group B at 1 month (p < 0.001) and 3 months (p = 0.003).
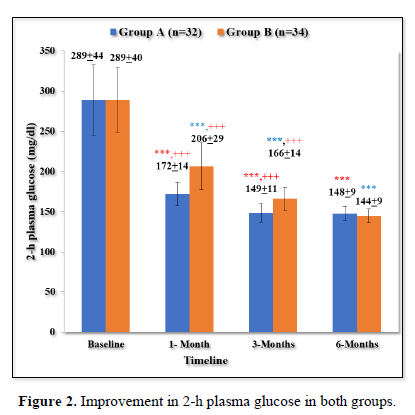
Improvement in 2-h plasma glucose in both groups
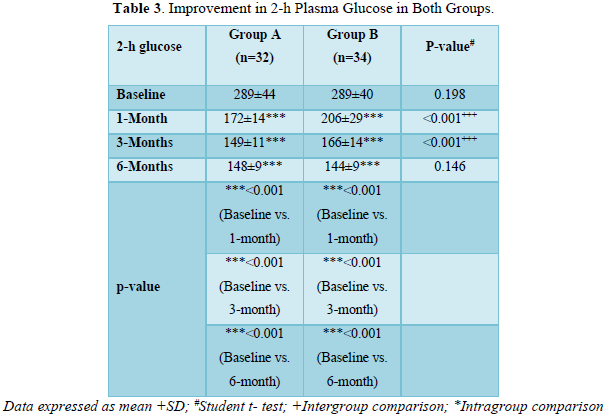
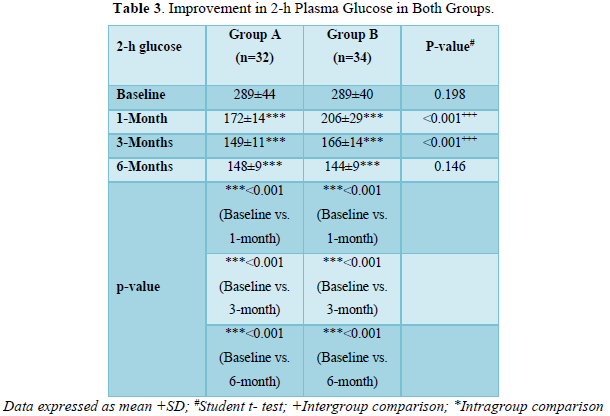
As shown in Table 3 and Figure 2, there was progressive significant decrease in 2-h plasma glucose in both the groups over 6 months. In Group A values improved from baseline of 289±44 mg/dl to 172±14 mg/dl (p < 0.001) at 1 month; 149±11 mg/dl (p < 0.001) at 3 months; 148±9 mg/dl (p < 0.001) at 6 months.
Similarly, In Group B values improved from baseline of 289±40 mg/dl (p < 0.001) to 206±29 mg/dl (p < 0.001) at 1 month; 166±14 mg/dl (p value < 0.001) at 6 months.
The patients in group A had statistically significant lower levels of 2- h plasma glucose in comparison to group B at 1 month (p < 0.001) and 3 months (p < 0.001). However, both the groups were comparable at baseline (p = 0.198) and 6 months (p = 0.146).
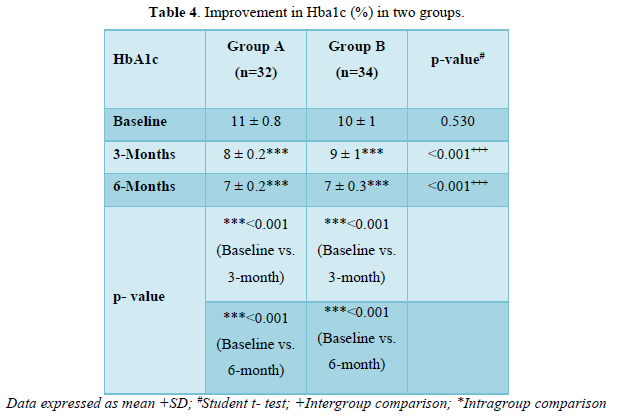
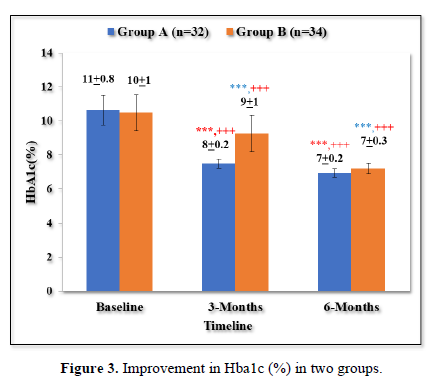
Improvement in HbA1c (%) in two groups
As shown in Table 4 and Figure 3 there was progressive significant decrease in HbA1c in both the groups over 6 months. In Group A values improved from baseline of 11±0.8% to 8±0.2% (p < 0.001) at 3 months and 7±0.2% (p < 0.001) at 6 months. Similarly, In Group B values improved from baseline of 10±1% to 9±1% (p <0.001) at 3 months and 7±0.3% (p < 0.001) at 6 months.
Both the groups were comparable at baseline (p =0.530). The patients in group A had statistically significant lower HbA1c levels in comparison to group B at 3 months (p <0.001) and 6 months (p <0.001).
DISCUSSION & CONCLUSION
Both the groups improved diabetes mellitus at the end of 6 months but empagliflozin was more effective in early follow up.
Similar observations were made by Haring Hans Ulrch [4], Inzucchi Silio E [7], Kawamori Ryuzo [5], and Hussain Mazhar [6].
LIMITATIONS
Being post graduate thesis, the follow-up could not be extended beyond 6 months. Follow-up for longer duration would have added more evidence about safety and efficacy of our study drugs.
FINANCIAL DISCLOSURE
No unnecessary financial burden was put on the patient for the treatment and investigations at any point of time throughout the study period. I did not get any financial benefit from any pharmaceutical company or any other source for this study.
CONFLICT OF INTEREST
No conflict of interest pertaining to any part of the study.
- Alvin CP, Kevin NDR, Evans MC (2018) Diabetes Mellitus: Diagnosis, Classification, and Pathophysiology. In: Jameson J. Larry, Kasper Dennis L, Longo Dan L, Fauci Anthony S, Hauser Stephen L, Loscalzo Joseph, Editors. Harrison Principles of Internal Medicine. 20th McGraw-Hill Education. pp: 2850.
- American Diabetes Association (2018) 8 Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes-2018. Diabetes Care 41(1): S73-S85.
- American Diabetes Association (2021) 9 Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes 2021. Diabetes Care 44(1): S111-S124.
- Häring HU, Merker L, Seewaldt-Becker E, Weimer M, Meinicke T, et al. (2014) EMPA-REG MET Trial Investigators. Empagliflozin as add-on to metformin in patients with type 2 diabetes: aA 24-week, randomized, double-blind, placebo-controlled trial. Diabetes care 37(6): 1650-1659.
- Kawamori R, Haneda M, Suzaki K, Cheng G, Shiki K, et al. (2018) Empagliflozin as add‐on to linagliptin in a fixed‐dose combination in Japanese patients with type 2 diabetes: Glycaemic efficacy and safety profile in a 52‐week, randomized, placebo‐controlled trial. Diabetes Obes Metab 20(9): 2200-2209.
- Hussain M, Elahi A, Iqbal J, Ghafoor MB, Rehman H, et al. (2021) Comparison of Efficacy and Safety Profile of Sodium-Glucose Cotransporter-2 Inhibitors as Add-On Therapy in Patients with Type 2 Diabetes. Cureus 13(4): e14268.
- Inzucchi SE, Davies MJ, Khunti K, Trivedi P, George JT, et al. (2021) Empagliflozin treatment effects across categories of baseline HbA1c, body weight and blood pressure as an add‐on to metformin in patients with type 2 diabetes. Diabetes Obes Metab 23(2): 425-433.




