Review Article
Crucial Role of the Immune System in Cancer Metastases Control and Therapeutic Implications
964
Views & Citations10
Likes & Shares
The humeral as well as the cellular immune system play an important role in cancer control. The therapeutic efficacy of immunological approach is limited by a restricted availability of tumor-specific antigens. Also, the efficacy of cytokine therapies and cell-based approaches (T-cells, dendritic cells, natural killer cells) is presently investigated in pre-clinical and clinical trials. In addition, several monoclonal antibodies have been approved by the U.S.A. Food and drug administration (FDA) for the treatment of system on the immunomodulation of cancer metastases and its crucial therapeutic role for improving the management of the disease. It also opens new avenue for a novel strategy of a promising cancer therapy based on the immune system of the patients suffering from the disease.
Keywords: Immune system, Cancer metastases, Immunotherapy
ROLE OF IMMUNE SYSTEM IN CANCER THERAPY
The immune system plays a key role in cancer progression control. The concept of cancer immune editing explores the hypothesis that cellular immunity promotes tumor growth and can also eradicate the disease [1,2]. As a matter of fact, immunotherapy (IT) is a type of biological therapy that depends on immune system to selectively destroy cancer cells. There are different subtypes of IT that presented by antibody-based therapy and cell-based therapy [3]. The former is presented by check inhibitors (CPIs) such as cytotoxic T-Lymphocytes associated proteins -4(CTLA-4), Programmed cell death protein-1 (PD-1) and programmed cell death protein ligand 1 (PD-L-1) [4] the second is presented by generating lymphocytes populations mainly T Cells that possess anti-cancer immune response. Generally, T-lymphocytes can be grouped into CD4+ helper T cells and CD8+ Cytotoxic T cells. They recognize their targets via the T-Cell receptors (TCRs), but after primary stimulation by antigen presenting cells presented by dendritic cells [5].
However, one of the biggest problems to find a cure for most solid malignancies is not the removal of the primary tumor, but the elimination of metastases [6]. The latter arise from solitary solid tumors when cancer cells undergo distinct changes and progress through a multi-step metastatic cascade, creating disseminated secondary tumors that difficult to treat. However, potentially immunogenic cancer cells could be recognized and destroyed by the host immune system [7]. Since, these cells acquire a high number of mutations and alterations that make them expressing tumor specific antigens that can be recognized as a non-self-antigen and thereby activate the immune system, eventually leading to destroying cancer cells [8].
As a matter of fact, there are two important players that are operating in killing cancer cells. These are CD8+ cytotoxic lymphocytes (adoptive immune system) and Natural Killer cells (innate immune the former firstly need to be activated and primed by recognition of tumor associated antigens, presented by antigen presenting cells (APCs) such as DCs. Meanwhile the second (NK cells) do not recognize tumor specific antigens and therefore do not need to be primed. They directly recognize cancer cells through antigen specific receptors [9,10].
It has also shown that, there is increasing evidence of tumor lymphocytic immune infiltration in triple negative breast cancer that has been associated with improved disease-free and overall survival rates in this subtype of breast cancer with and without any treatment [11].
Furthermore, adoptive cell therapy (ACT) utilizing endogenous tumor-infiltrating lymphocyte (TILs) has shown a complete response lasting beyond 3 years in a 20% of metastatic melanoma patients [12] Also, ACT with TILs was found to be a useful therapeutic strategy in the treatment of human pancreatic tumors with high lethality and limited treatment options [13].
Moreover, it has been documented that, the decreased ratios of CD8+Cytotoxic T lymphocytes to FoxP3+CD4+CD25+ (T-Reg cells) in tumors correlate with poor prognosis among cancer patients [14].
It has also been shown that, Tumor-infiltrating T cells, (CD8+) / T-Reg cell ratio and CD8+/ cancer stem cells ratio are correlated with lymph node metastasis in patients with early breast cancer [15].
It is well known that, cellular immunotherapies approach encompasses multiple strategies, including the adoptive transfer of tumor-infiltrating lymphocytes, dendritic cells, natural killer cells and engineered immune components such as chimeric antigen receptors constructed T-cells (CAR-T cells) and engineered T cell receptors [16] (Figures 1 & 2).
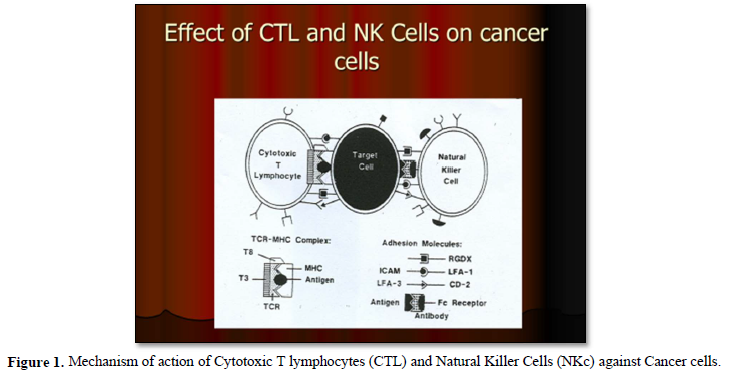
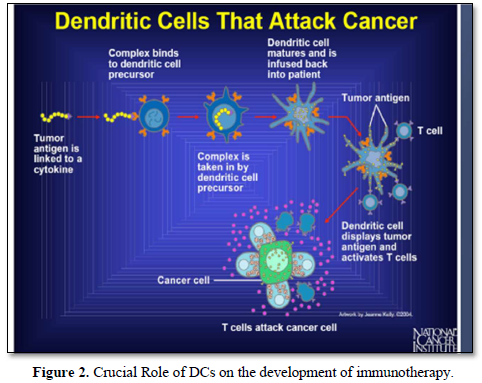


It has been also shown that, treatment with immunotherapy using Anti-CTLA-4, Anti-PD-1 or the combination of these antibodies was very promising results and able to improve survival in patients with metastatic melanoma. Also, adoptive immunotherapeutic strategy using tumor- infiltrating lymphocytes has shown very promising results in those previous mentioned patients [17].
ROLE OF IMMUNE SYSTEM IN CANCER VACCINES
As a matter of fact, Dendritic cells (DCs) represent an ideal effective strategy for the design an effective vaccine for cancer patients. Recent advancement on the knowledge of the numerous DCs subtypes, their functions and T-cell polarizing abilities has led to the development of several protocols for the ex-vivo different ion of autologous DCs and Their loading with tumor-associated antigens. Also, novel strategies for in-vivo targeting of the latter and adjuvants to natural DCs subsets have been developed [18].
It has been shown that, he efficacy of cancer vaccines strategies have difficulties to generate broad and robust immune responses as well as to overcome immune escape mechanisms [19]. The preclinical and clinical studies have demonstrated that for optimal induction of tumor-specific T cells, DC vaccines should exhibit three major criteria,
- First: an ability to migrate to lymph nodes
- Second: the DCs must maintain its mature phenotype in the lymph nodes for sufficient time to activate and expand a tumor-specific T-cell response that is able to eliminate the tumor.
- Third: the DCs must stably present Tumor associated antigens [20].
Moreover, an effective DC vaccine for malignancies must provide cognate T- cells with three signals as follow:
- First Signal: antigen presented as peptide epitopes on MHC molecules
- Second Signal: Membrane-bound costimulatory molecules such as CD80 and CD86.
- Third Signal: Soluble co-stimulatory molecules such as the pro-inflammatory Cytokine IL12 [21].
As a matter of fact, DCs cancer vaccines aim to stimulate the anti-tumor immunity in the patients by harnessing the capacity of DCs to activate tumor-specific T-cells This can be performed by infusing patients with ex-vivo antigen loaded DCs [22]. Also, DCs play a pivotal role in the tumor micro environment, so tumor infiltrating DCs could open new avenue for a promising development in cancer therapeutic strategy [23] (Figure 3).
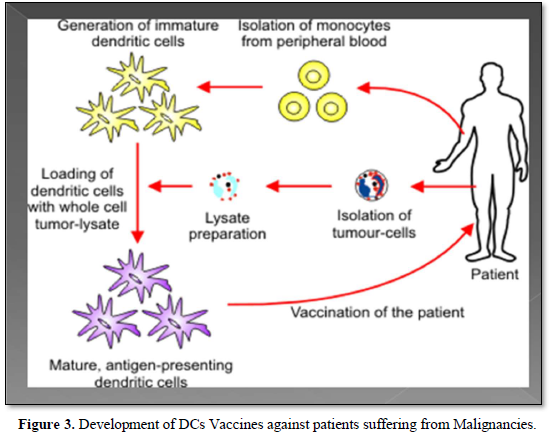

Moreover, the clinical benefit of the therapeutic cancer vaccines has been established, that was mostly noted as prolonged survival among those cancer patients treated with Cancer vaccines. It has been also observed that, the specificity of therapeutic vaccination combined with some immunomodulation such as T cell checkpoint inhibitors could offer an attractive avenue for the development of future cancer therapy [24].
Furthermore, regarding the techniques that are using for the current cancer vaccines could fall into the following three major categories;
- First: cell vaccines (Tumor or immune cells)
- Second: Protein/peptide vaccines and finally
- Third: nucleic acid Vaccines (DNA, RNA, or viral vector) [25].
It has been demonstrated that, anti-HER-2 DCs vaccination is a safe and immunogenic treatment to induce tumor-specific T cell responses in HER-2 positive patients [26].
DC vaccination in patients could be more effective when combined with other therapies or agents with immune modulatory function. In phase I Clinical trial of DC Vaccination for recurrent ovarian cancer, patients were treated with DC vaccination alone DC vaccine plus bevacizumab or DC vaccine with the latter plus low-dose of Cyclophosphamide given the day before the vaccination [27]. Moreover, Sipuleucel-T Vaccine derived from peripheral blood leukocytes treated with a fusion protein composed of the prostate tumor antigen (prostatic acid phosphatase) combined with granulocyte mononuclear colony stimulating factor (GM-CSF) to promote DCs differentiation from precursor monocytes was approved by U.S.A FDA for treatment of metastatic Castration-resistant prostate cancer [28].
ROLE OF IMMUNE SYSTEM IN CANCER METASTASES CONTROL
One of the biggest problems to find an effective cure for most solid malignancies is not the removal of the primary Tumor but the elimination of its metastases. Therefore, the latter are the leading cause of death among patients suffering from malignancies [29].
Over a century ago, experiments have indicated a link between the immune system and cancer metastases. Those previous studies have shown that; the primary malignant tumor (PMT) could induce an immune response, which may not be sufficient to destroy the PMT, but could prevent the growth of secondary tumor or metastases [30] (Figure 4).
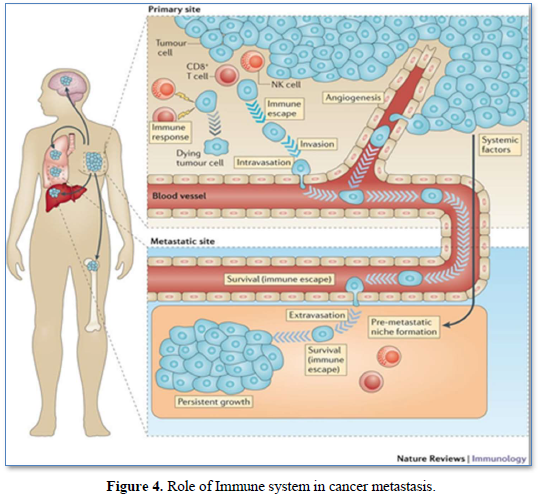

It was also shown that, murine models of metastasis revealed a progressive growth of primary tumor suppressed the growth of a newly implanted secondary tumor through the immune system mechanism, a phenomenon known as concomitant immunity (CI). In another word, the tumor can induce both an anti-tumor immune response as well as immunosuppressive mechanism to evade an attack by immune system [31]. Recent studies have provided a deeper under-standing of the impact of surgical tumor resection on the systemic immune state and immunological control of metastasis. The phenomenon of the metastatic outgrowth following surgical tumor resection has been documented in several cancer types, where shedding of tumor cells into the circulation of the patients lead to new and accelerated metastatic growth despite the resection of the primary tumor [32]. On the other hand, Surgical resection of the primary tumor triggers healing programs that elevate circulating IL-6 and G.M-CSF and ultimately drive myeloid subsets towards immunosuppressive states [33]. Moreover, Patients with colorectal cancer after surgical resection exhibited decreased IFN-g secretion from peripheral NK Cells compared to healthy subjects. Therefore, these data suggested multiple mechanisms by which surgical resection induces global immunological perturbation that can promote metastasis [34].
Recently, Treatment with immunotherapy namely anti-CTLA-4, anti-PD-1 or the combination of these anti-bodies, also adoptive cell therapy using tumor-infiltrating lymphocytes have shown very promising results and were able to improve survival in patients with metastatic melanoma [35]. It has been shown that, tumor-infiltrating lymphocytes are an important component of the tumor microenvironment in breast cancer. Also, tumor- infiltrating regulatory T-Cells, CD8+/T-Reg. Ratio and cancer stem cells are correlated with lymph node metastasis in patients with an early breast cancer [36].
As a matter of fact, metastasis is the leading cause of death among cancer patients. During the metastatic cascade, cancer cells tightly interact with the immune system and they influence each other, both in the tumor microenvironment and systemically [37].
It has been shown that, immune cells are important regulators of angiogenesis and lymph angiogenesis [38]. The latter are promoted by hypoxia in the tumor micro-environment. Hypoxia in turn influences the recruitment and function of tumor infiltrating immune cells in promoting their pro-angiogenic function [39].
CONCLUSION
Immune system could open new avenues for the development of novel biological therapy, vaccines and control of metastases for those patients suffering from malignancies.
- Zitvogel L, Aptoh L, Ghiringhelli F, André F, Tesniere A, et al. (2008) The anticancer immune response indispensable for therapeutics success. J Clin Investig 118(6): 1991-2001.
- Dunn PG, Bruce AT, Ikeda H, Old LJ, Schreiber RD, et al. (2002) Cancer immune editing: From immune surveillance to tumor Escape Nat Immunol 3(11): 991-998.
- Altman DM (2018) A Nobel prize-worthy pursuit cancer immunology and harnessing immunity to tumor neoantigens. Immunology 155(3): 283-284.
- Gong J, Chehrazi-Raffle A, Reddi S, Salgia R (2018) Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy. A comprehensive review of registration trials and future considerations. J Immunol Cancer 6(1): 8.
- Kruger S, Ilmer M, Kobold S, Cadilha B L, Endres S, et al. (2019) Advances in cancer Immunology latest trends. J Exp Clin Cancer Res 38: 268.
- Weigelt B, Peterse JL, Van’t Veer LJ (2005) Breast Cancer metastasis, markers and models Nat Rev Cancer 8: 591-602.
- Dunn GP, Old LJ, Schreiber RD (2004) The immunobiology of Cancer, immunosurveillance and immunoediting. Immunity 2: 137-148.
- Chen DS, Mellman I (2013) Oncology meets immunology: The cancer-immunity cycle. Immunity 39(1): 1-10.
- Hofstad T (1977) Cross-reactivity of Bacteroides Fragilis O antigens. Acta Pathol Microbiol Scand B 85B(1): 9-13.
- Ruggiero RA, Bustuoabad OD, Bonfil RD (1985) Concomitant immunity in murine tumors of non-detectable immunogenicity. Br J Cancer 51(1): 37-48.
- Teijido PG, Cabal ML, Fernandez IP, Perez YF (2016) Tumor- infiltrating Lymphocytes in triple Negative Breast Cancer; The future of immune Targeting. Clin Med Insights Oncol 10(S1): 31-39.
- Rosenberg SA, Yang JC, Sherry RM, Dudley ME (2011) Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 17(13): 4550-4557.
- Maclean H, Hao L, Molène M, Sanaik AA (2016) Expansion of tumor-infiltrating Lymphocytes (TILs) from Human pancreatic tumors. J Immunother Cancer 4: 61.
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, et al. (2004) Specific recruitment of regulatory T cells (T-Reg.) in ovarian Carcinoma fosters immune privilege and predicts reduced Survival. Nat Med 10(9): 942-949.
- Luis AC, Gina SG, Alexander PG (2020) Tumor-infiltrating regulatory T cells, CD8+/ T-Reg. Ratio and Cancer stem cells are correlated with lymph Node metastasis in patients with early breast cancer. Breast Cancer 27: 837-849.
- Antrás JF, Hoyer KG, Piqué MB, García-Sáenz JÁ, Pérez-Segura P (2018) Adoptive cell therapy in breast cancer: A current perspective of next generation Medicine. Front Oncol 10: 605633, 16: 3045-3053.
- Foppen GMH, Donia M, Svane IM, Haanen JBAG (2015) Tumor -infiltrating lymphocytes for the treatment of metastatic Cancer. Mol Oncol 9: 1918-1935.
- Bracci L, Cpone I, Moschella F, Proietti E, Belardelli F (2013) Exploiting Dendritic Cells in the development of Cancer Vaccines. Expert Rev Vaccine 12(10): 1-6.
- Marme F (2016) Immunotherapy in Breast Cancer. Oncol Res Treat 39: 335-345.
- Turnis ME, Rooney CM (2010) Enhancement of Dendritic cells as vaccines for cancer. Immunotherapy 2(6): 847-862.
- Melief CJM, Hall T van, Arens R, Dssendorp Ferry, van der Burg SH (2015) Therapeutic Cancer Vaccines. J Clin Invest 125(9): 3401-3412.
- Le CMG, Weiden J, Eggermont LJ, Figdor CG (2018) Dendritic cells in Cancer Immunotherapy. Nat Mater 17: 472-477.
- Janco Jo MT, Lamichhane P, Karyampoudi L, Knutson KL (2015) Tumor- infiltrating Dendritic cells in Cancer Pathogenesis. J Immunol 194(7): 2985-2991.
- Igarashi Y, Sasada T (2020) Cancer Vaccines: Toward and the Next Breakthrough in Cancer Immunotherapy. J Immunol Res 582540: 1-13.
- Weigelt B, Peterse JL, Vant veer LJ (2005) Breast Cancer metastasis markers and models. Nat Rev Cancer 5(8): 591-602.
- Lowenfeld L, Mick R, Datta J, Czenieck BJ, Xu S, et al. (2016) Dendritic Cell vaccination enhances immune responses and induces Regression Of HER-2 positive DCIS Independent of Route: Results of Randomized Selection Design Trial. Clin Cancer Res 23(12): 2961-2971.
- Tanyi JL, Bobisse Sara, Ophir E, Tuyaerts S, Roberti A, et al. (2018) Personalized cancer vaccine effectively mobilizes anti-tumor T cell Immunity in ovarian cancer. Sci Transl Med 10(436): eaao5931.
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, et al. (2010) Sipuleucel-T immunotherapy for castration- resistant prostate cancer. N Engl J Med 363(5): 411-422.
- Janssen LME, Ramsay EE, Logsdon CD, Ovrwijk WW (2017) The immune system in Cancer metastasis: friend or foe? J Immunother Cancer 5(1): 79.
- Ruggiiero RA, Busuoadad OD, Bonfil RD (1989) Anti-tumor concomitant immunity, a possible metastasis control Mechanism. Medina (B Aires) 49(3): 277-281.
- Xue D, Xia T, Wang J, Chunhui Z, Chong M (2018) Role of Regulatory and CD8+ Lymphocytes in the dissemination of circulating tumor cells in primary Invasive Breast cancer. Oncol Lett 16: 3045-3053.
- Tohme S, Simmons RL, Tsung A (2017) Surgery for cancer: A trigger for metastasis. Cancer Res 77: 1548-1552.
- Krall JA, Reinhardt F, Mercury OA, Pattabiraman DR, Brooks MW, et al. (2018) The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy. Sci Transl Med 10(436): eaan3464.
- Angka L, Martel AB, Kilgour M, Jeong A, Sadiq M, et al. (2018) Natural killer cells INF-g secretion is profoundly suppressed Following colorectal cancer surgery. Ann Surg Oncol 25(12): 3747-3754.
- Foppen MHG, Donia M, Svane LM, Haanen JBAG (2015) Tumor- infiltrating Lymphocytes for the treatment of metastatic cancer. Mol Oncol 9: 1918-1935.
- Castillo LAS, Garcia-Romo GS, Pedroza-Gonzalez A, Diaz-Rodriguez A, Reyes-Hernandez D, et al. (2020) Tumor -infiltrating regulatory T cells, CD8+/T Reg Ratio and Cancer stem cells are correlated with lymph node metastasis in patients with early breast cancer. Breast Cancer 27: 837-849.
- Blomberg OS, Spagnuolo L, de Visser Karin E (2018) Immune Regulation of metastasis: Mechanistic insights and therapeutic Opportunities. Dis Model Mech 11(10): dmm036236.
- Alitalo K, Tammela T, Petrova TV (2005) Lymph angiogenesis in development and human disease Nature 438: 946-953.
- Kumar V, Gabrilovich DI (2014) Hypoxia- inducible factors in regulation of immune responses in tumor microenvironment. Immunology 143: 512-519.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- International Journal of Medical and Clinical Imaging (ISSN:2573-1084)
- Journal of Neurosurgery Imaging and Techniques (ISSN:2473-1943)
- International Journal of Radiography Imaging & Radiation Therapy (ISSN:2642-0392)
- Chemotherapy Research Journal (ISSN:2642-0236)
- Journal of Ageing and Restorative Medicine (ISSN:2637-7403)
- Journal of Oral Health and Dentistry (ISSN: 2638-499X)
- Journal of Rheumatology Research (ISSN:2641-6999)





